Seizures in the neonate
exp date isn't null, but text field is
Objectives
This guideline is applicable to doctors, nurses and ANNPs working with neonates in the West-of-Scotland managed clinical network. This guideline is intended to provide the following guidance on identification, aetiology, investigation and management of neonatal seizures. This guideline should be used with the appropriate pharmacy monographs.
The risk of having a seizure is greatest during the first year of life, with the highest incidence in the neonatal period.(1-3). It is therefore important to recognise neonatal seizures, establish their aetiology and to manage them. Seizures in the newborn frequently signal significant brain pathology such as hypoxic ischaemic injury, infection, stroke, inborn error of metabolism, intracranial haemorrhage, drug withdrawal or brain malformation. (3,4) There is evidence that both clinical and electrographic seizures are associated with adverse neurological sequelae and an increased risk of epilepsy later in life. (5-7)
The exact incidence of neonatal seizures in the general newborn population is difficult to estimate. Traditionally the diagnosis of seizures was made by clinical observation of abnormal movements, some of which were not seizures, leading to an over estimation of seizure activity. However electrographic seizures are not always manifested clinically which in turn can lead to an underestimation of seizure frequency.(3) It is thought the incidence of seizures in infants born at term is 1- 3 per 1000 live births.(3,8-10) The incidence is much higher in preterm and low birth weight (<1500g) neonates ranging from 57.5 – 132 per 1000 live births. (9,11-14) Recognition of seizures is more frequent with the use of continuous EEG monitoring.(9,13)
Seizures represent an underlying disease process due to a disturbance of the electrical activity of the brain.(3,15) The developing brain is very susceptible to seizures and the mechanisms are complex and not well understood. (15) It is thought that the immature brain is more prone to seizures because of characteristics of the neurons, neurotransmitters, receptors, synapses, myelination, glia, cellular function and neuronal circuitry(16). The increased susceptibility to seizures is also thought to be related to an imbalance between increased cellular and synaptic excitation and lack of inhibitory receptors and neurotransmitters, mainly related to GABA. (13,15,17,18)GABA is usually an inhibitor but in the immature neonatal brain it has an excitatory effect. This effect changes around term in primates and with progressive development it adopts a neuro-inhibitory role. (18)
In cases of neonatal seizures an underlying cause can be found in 75 to 90%.(19) There are many causes for seizures in neonates but hypoxic ischaemic encephalopathy (HIE), intracranial haemorrhage, intracranial infection and developmental defects account for up to 85% of neonatal seizures. (3,9,20) HIE is the most common underlying condition causing seizures in term infants and intraventricular haemorrhage accounts for the majority of seizures in infants < 30 weeks gestation. (3,9)
The International League Against Epilepsy (ILAE) have proposed a framework for classifying the aetiology of neonatal seizures, adapted from the ILAE classification of the epilepsies. (48) This will allow for more uniform classification of aetiology.

Figure 1: Common aetiologies of neonatal seizures in term infants from The ILAE classification of seizures and the epilepsies modification for seizures in the neonate. Proposal from the ILAE task force on neonatal seizures. (48)

Figure 2: Framework for neonatal seizures adapted form 2017 ILAE Framework of the epilepsies from The ILAE classification of seizures and the epilepsies: modification for seizures in the neonate. Proposal from the ILAE task force on neonatal seizures. (48)
* including perinatal hypoxic ischaemic encephalopathy and other hypoxic events in the neonatal period
** including infarction, haemorrhage, brain trauma and brain malformations
Table 1. Aetiology of Neonatal Seizures - NB this list is not exhaustive and where a cause is not identified, consultation with Neurology and Genetics is recommended
|
CAUSE |
|
|
HYPOXIC |
|
|
Hypoxic Ischaemic Encephalopathy (HIE) |
|
|
STRUCTURAL |
|
|
Intracranial Haemorrhage
|
|
|
Perinatal Stroke |
|
|
Congenital Malformations of Cortical Development |
Associated with poor neurodevelopmental outcome |
|
INFECTIOUS |
|
|
Central Nervous System Infection |
Account for 5-10 % of seizures (3)
|
|
METABOLIC |
|
|
Metabolic |
|
|
Vitamin Responsive Seizures (3) |
|
|
Inborn Errors of Metabolism |
|
|
GENETIC |
|
|
Severe epilepsy syndromes |
|
|
Genetic Epilepsies |
|
|
Benign Non-familial Neonatal Convulsions (Fifth day fits) |
|
|
Benign Familial Neonatal Seizures |
|
|
UNKNOWN/OTHER |
|
|
Neonatal Abstinence Syndrome (NAS) |
|
|
Maternal Non-narcotic Drug Use |
|
Any unusual or stereotypical movement may represent a seizure but there some normal movements that may be mistaken for seizure activity in the newborn infant. It is therefore important to differentiate between the two to avoid over treatment of normal movements. A video of events can help to identify if the events are epileptic or non-epileptic in nature.
There are many non-epileptic events which can be seen in the neonatal period which can mimic epileptic events. These include:
- Jitteriness (video link to be inserted here)
- Benign neonatal sleep myoclonus (video link to be inserted here)
- Motor automatisms (video link to be inserted here)
- Reflux/Sandifer syndrome (video link to be inserted here)
- Hyperekplexia (video link to be inserted here)
NB – these videos are unavailable currently – we will add a link as soon as they are online
Jitteriness is a common finding in the newborn baby, occurring in up to two-thirds within the first three days of life.(30) It is usually a benign finding but can be associated with hypoglycaemia, hypocalcaemia, sepsis, HIE and drug withdrawal. Jitteriness is associated with a rapid, tremulous movement in one or more limbs usually in response to minor stimulation. These movements stop when held unlike in a seizure where the movements would continue despite restraint. (13)
Benign neonatal myoclonus occurs during sleep and is usually seen in the upper limbs. It can be distinguished from epileptic myoclonus by the fact that the jerks occur only during sleep and settle on arousal. (30) It is not associated with any adverse effects. (3)
An epileptic seizure is defined as a seizure with EEG correlate. ILAE propose defining neonatal seizures as “an electrographic event with a pattern characterised by a sudden, repetitive, evolved stereotyped waveforms with a beginning and end”. (48)
The ILAE Task Force on Neonatal Seizures has published a draft framework on the classification of neonatal seizures which will become the ILAE position on classification on seizures in the neonatal population. (48) The use of EEG in diagnosis of seizures is emphasised.
It is worth noting that generalised tonic-clonic seizures are rare in the first month of life due to the immature myelination of the nervous system and are not seen in premature infants. Neonatal seizures tend to be focal in nature and are usually of short duration.
Figure 3: Proposed diagnostic framework of seizures in the neonatal period, including classification of seizures from The ILAE classification of seizures and the epilepsies: modification for seizures in the neonate. Proposal from the ILAE task force on neonatal seizures. (48)
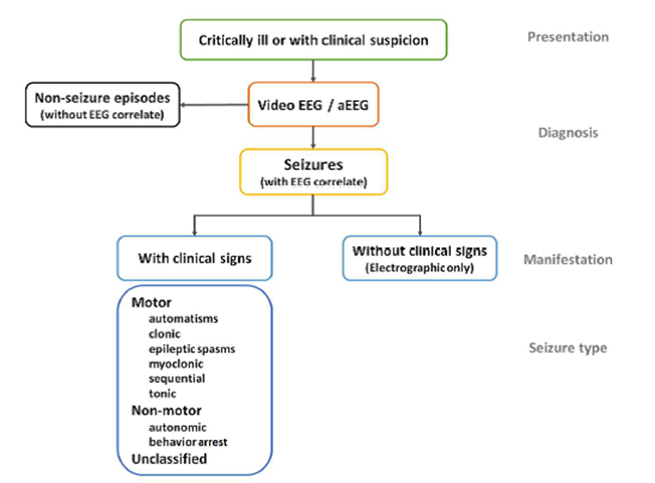
Automatisms:
- a more or less coordinated motor activity which may resemble a voluntary movement
- typically oral
Clonic:
- repetitive jerking involving the same muscle groups
- can be symmetrical or asymmetrical
Epileptic spasms:
- a sudden flexion, extension or mixed extension-flexion of predominantly proximal and truncal muscles
- more sustained than a myoclonic movement
- may occur in clusters
- may present as head nodding, grimacing or subtle eye movements
- rare
Myoclonic:
- a sudden, brief, involuntary single or multiple contraction(s) of muscle(s) or muscle groups
- can be difficult to differentiate from non-epileptic myoclonus.
Sequential:
- events with a sequence of signs, symptoms and EEG changes at different times with no predominant feature determinable as several features occur in sequence with changing lateralisation within or between seizures
Tonic:
- a sustained increase in muscle contraction
- usually focal, unilateral or bilateral asymmetric.
Autonomic:
- a distinct alteration of autonomic nervous system function involving cardiovascular, pupillary, gastrointestinal, sudomotor, vasomotor and thermoregulatory functions
- may involve apnoeas
- typically seen with other seizure manifestations
- requires EEG confirmation that it is a seizure
Behavioural arrest:
- a pause in activities, freezing
- may be focal and/or followed by an apnoea, other autonomic features and motor seizures
Unclassified:
- inadequate information or unusual clinical features resulting in an inability to classify the seizure under the other categories.
Detailed maternal history
- Maternal diseases
- Prothombotic disorders, infections, diabetes, seizures (esp. in infancy)
- Family History
- Epilepsy, inborn errors of metabolism
- Maternal drug use
- Anti-depressants – e.g. SSRIs – fluoxetine, citalopram
- Illicit drugs – e.g. Methadone, cocaine
- Alcohol
- Consanguinity
- Pregnancy History
- Previous miscarriages
- Pre-eclampsia
- IUGR
- Unusual fetal movements
- When first noticed, how often?
- History of fetal “hiccups”?
- Reduced fetal movements
- History of GBS or prolonged rupture of membranes?
- Birth History
- Fetal heart rate monitoring, cord blood gases, Apgar scores looking for signs of birth asphyxia
- Mode of delivery
- Seizure history
- Age at onset, frequency
- Type of seizure – classified by predominant clinical feature
- Associated bradycardia/apnoea/cyanosis?
- Conscious/sleeping/unconscious?
- Eye movements/position
- Condition of baby prior to seizures - Lethargic, poor feeding, vomiting, jaundiced?
Any infant who has a seizure should have a thorough clinical examination performed.
- Cardiovascular monitoring
- Respiratory rate, oxygen saturations, heart rate, blood pressure, temperature
- General
- Dysmorphic features, jaundice, pallor, OFC, unusual odour
- Skin
- Rashes, birth marks, bleeding, bruising, evidence of neuro-cutaneous syndromes
- Neurology
- Consciousness
- Anterior fontanelle
- Micro/macrocephaly
- Tone, reflexes, posture, movements
- Cardiovascular and Respiratory
- Murmurs including auscultating the anterior fontanelle
- AV Malformation
- Work of breathing
- Murmurs including auscultating the anterior fontanelle
- Abdominal
- Masses – liver, kidney, spleen
It is important to carry out frequent examinations of the infant looking for changes in clinical signs that may point to the underlying cause or for deterioration in their condition.
First line investigations should be performed in all infants who present with seizures. If clear case of HIE or sepsis then it may not be necessary to perform the genetic or metabolic investigations. For infants in which seizures persist despite treatment with an anti-epileptic and the cause remains unclear, then second line investigations should be carried out.
First Line – for all infants with seizures
Plasma
- Blood glucose
- Urea and Electrolytes (UE) – sodium, potassium, calcium and magnesium
- Blood gas – acid base, lactate, bicarbonate, CO2
- Liver function tests (LFT) - ALT/ALT, bilirubin
- Full blood count (FBC)
- Blood cultures
- Virology – HSV, CMV, adenovirus, EBV
- CRP – first CRP may not be raised, serial CRPs are more informative
Urine
- Culture – bacterial
- Virology – CMV
CSF
- Bacterial Culture
- Virology – HSV, VZV, enteroviruses
- Glucose
- Protein
Imaging(15,19,31)
- Cranial Ultrasound
- Readily and rapidly available in most neonatal units.
- Useful first line investigation to look for IVH and malformations.
- In more experienced users it can be used to identify neonatal strokes and infections.
Other
- Throat swab – CMV (viral medium)
- Bacterial and viral swabs of any skin lesions or sticky eye
Additional investigations if cause not clear from history
(i.e. HIE or sepsis) then include the following as part of first line investigations
Plasma
- Genetic investigations – karyotype, array CGH, epilepsy panel (100+ genes associated with epilepsy); when performing initial investigations, 1ml EDTA should be taken and sent for storage of DNA which can be used to identify genetic causes of seizures on the neonate should imaging and initial investigations not identify seizure aetiology. Discussion with Genetics or Neurology may be useful in determining whether single gene or gene panel investigation would be most useful. NB - A referral to clinical genetics should be considered if these investigations are considered
- Metabolic investigations – plasma amino acids, ammonia, lactate (if not available on blood gas), newborn screening
Urine
- Metabolic – amino and organic acids
CSF
- Lactate
- Amino acids (specifically glycine and serine)
If the above investigations do not identify the cause or if there are atypical features then the following may be considered after discussion with metabolic specialist / neurologist
Plasma
- Pipecolic acid and gene test for antiquitin (pyridoxine deficiency)
- Very long chain fatty acids ( peroxisomal dysfunction)
Urine
- Alpha-amino adipic semialdhyde (AASA) ( pyridoxine deficiency) – not altered by previous administration of pyridoxine
- Urinary sulphites (fresh urine sent to biochemistry rapidly) ( Molybdenum Co Factor Deficiency)
CSF
- Pyruvate
- Neurotransmitters – need to arrange with Biochemistry 24hrs in advance to obtain the appropriate specimen sample bottles and dry ice
Imaging(15,19,31)
- MRI
- ‘Gold Standard’ in the examination of the newborn brain
- Can identify pathology such as HIE, arterial and venous stroke, meningitis/encephalitis, brain malformations
- All infants who are treated for HIE should have an MRI performed in accordance with the Cooling Guideline
- To determine the optimal timing for MRI refer to the Colling guideline where the underlying diagnosis is HIE, otherwise discuss with a radiology consultant
Neurophysiology - depending on local availability and expertise, may require transfer when clinically stable (19,32-34)
- Cerebral Function Monitoring (CFM)/ Amplitude-integrated EEG (aEEG)
- Bedside monitor that allows us to gather information on the electrical activity of a sick neonate’s brain. It can help in identifying or confirming seizure activity and the response to treatment
- Bedside monitor that records electro-cortical activity from a limited number of channels, usually 1 or 2, to give a filtered, compressed, amplitude-integrated EEG (aEEG)
- A single channel raw EEG can be viewed to confirm that changes in the aEEG are seizure activity
- Electroencephalogram (EEG)
- Limited availability, as a consequence rarely performed acutely unless on site availability.
- Full 20 lead EEGs with concurrent video are most sensitive in detecting seizures
- Provides confirmation that any observed movements/phenomena are seizures
- Not all clinical seizures are detected by EEG, often called “electro-clinical dissociation”(8)
- Not all seizures noted on the EEG correlate with abnormal movements, these are known as subclinical seizures
- See guideline on the use and interpretation of the CFM monitor
- See appendix for examples of CFM readings
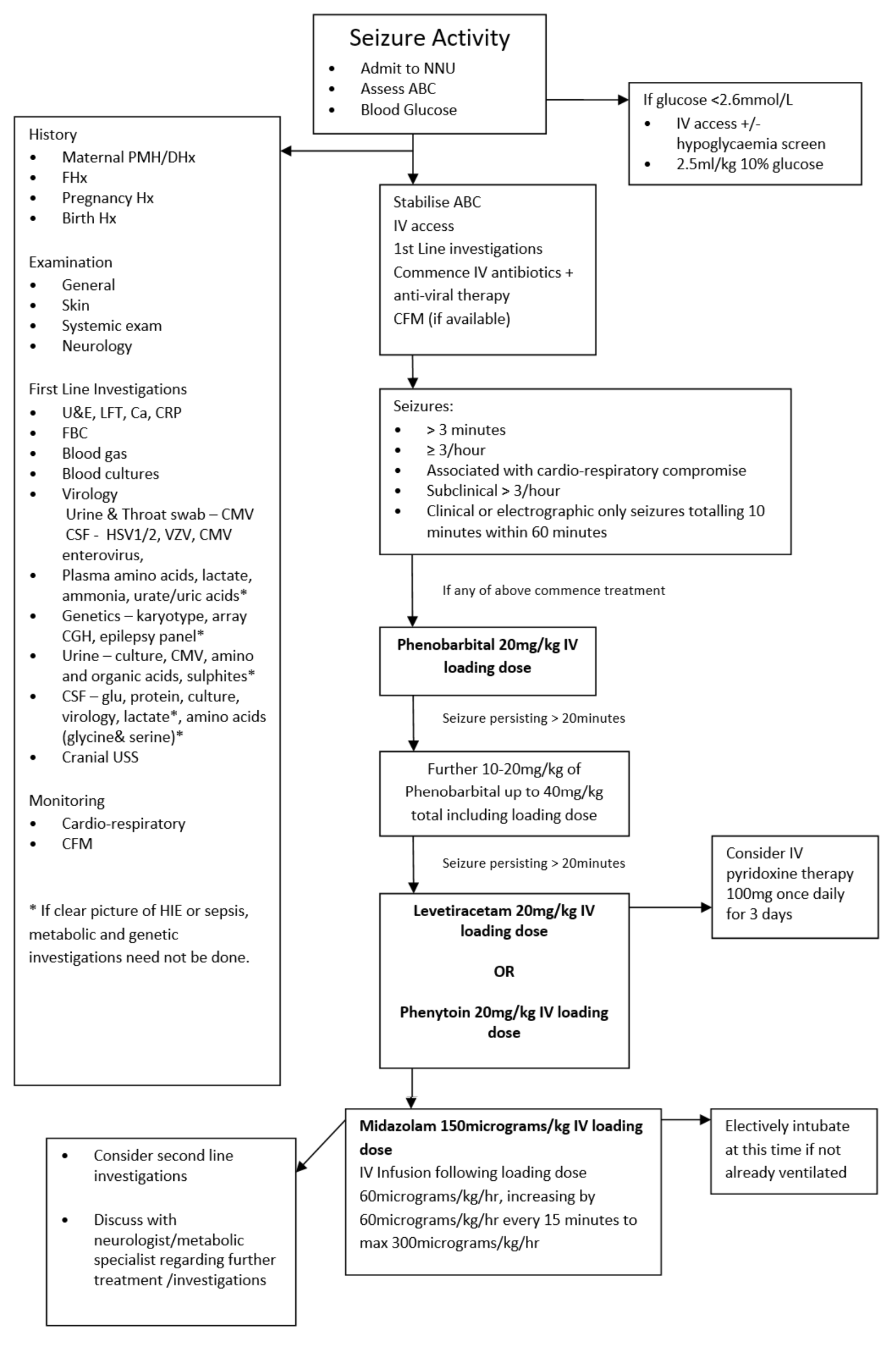
There is growing evidence that neonatal seizures can contribute to a poor neurodevelopmental outcome so prompt recognition, investigation and instigation of treatment is required. It is important to look for easily detectable underlying causes like hypoglycaemia or electrolyte disturbances as these are relatively straight forward to manage and may prevent the need for anti-epileptic drugs (AED) and further brain damage by prolonged seizures. It is also important to consider sepsis or encephalitis as a cause and commence empiric antibiotics and antiviral therapy.
Initial Management
It is important in any infant having seizures to ensure that they are cardiovascularly stable and initial management should include assessment and management of:
- Airway
- Breathing
- Circulation
- Blood Glucose
The infant should be admitted to the neonatal unit, if not already, for observation and have full cardiovascular and respiratory monitoring and if available locally, CFM monitoring attached. First line investigations should be initiated to try and identify an underlying cause and guide management. If considering an inborn error of metabolism it is important to stop feeds until the diagnosis has been excluded. It is also important to inform the consultant of any baby on the unit developing seizures or of any admission of a baby with seizures.
When to Treat
There is great uncertainty about when to commence treatment for neonatal seizures. There is even more uncertainty about when to treat clinical seizures without EEG correlate and also subclinical seizures. (8) A general consensus is to treat seizures that are:(13)
- Prolonged – seizure lasting >3 minutes (Treatment may commence before this if other criteria are met)
- Frequent – 3 or more seizures per hour
- Associated with cardiovascular disturbance
- Subclinical - > 3 sub-clinical seizures on CFM within 60 minute period
- As per HIE guideline, if seizure activity (clinical or electrical) equates to more than 10 minutes in a 60 minute period
Anti-Epileptic Drugs
There is a lack of high quality evidence regarding which AED to use. A Cochrane review in 2004 by Booth and a systematic review in 2013 by Slaughter et al both concluded that there is little evidence from RCTs to support the use of any anti-convulsant drugs (AED) currently used in the neonatal period and that further research is required. (4,14) Phenobarbital and phenytoin are the most commonly used first and second line AEDs, despite being shown to be relatively ineffective. Painter et al carried out an RCT comparing the two as first line treatments and found that less than 50% of the patients had seizures cessation in either group. In the same study if seizure control was not achieved addition of the other drug only increased the seizure cessation rate to 60%. (35) Small case series have reported successful use of midazolam in neonates with seizures refractory to phenobarbital, with or without the addition of phenytoin. (36-38)
For all the below medications please see the Pharmacy monographs for guidance on the preparation and administration.
Phenobarbital – First Line
Phenobarbital continues to be the first line AED used for the treatment of neonatal seizures. Phenobarbital prolongs and potentiates the action of GABA, an inhibitory neurotransmitter. (1) In a recent phase IIb study comparing phenobarbital to levetiracetam, phenobarbital was found to be more effective at controlling seizures that levetiracetam. (55)
- For loading dose of Phenobarbital see WoS drug monographs, administer over 20 minutes. NB The maximum anticonvulsant effect may take up to 20 minutes following completion of a Phenobarbital infusion
- If full seizure control is not achieved following the initial loading dose, further one or two smaller loading doses of Phenobarbital may be given, see WoS drug monographs.
- If there is no reduction in seizure activity and the infant is physiologically compromised preparations should be made to administer a second line agent – see below.
- Measure level 3 hours after completing loading dose
- Therapeutic range 15-40mg/L
- Half-life 3-8 days
- Can cause electro-clinical dissociation (41)
- Clinical manifestations of seizures cease due to sedative effect of phenobarbital but electrical seizures continue
- Maintenance dose not usually required
- If required see pharmacy monograph for doses.
Phenobarbital is metabolised by the liver and excreted by the kidney so toxicity can occur if there is any liver or kidney impairment. e.g. following ischaemic injury in perinatal asphyxia or therapeutic hypothermia. There is no requirement to adjust the dose in these circumstances but it should be noted that the sedative effects of phenobarbital may last longer in babies with asphyxia. There are some concerns about phenobarbital triggering apoptotic neurodegeneration and the subsequent impact of this on neurodevelopmental outcomes, but this remains the first line drug of choice in managing neonatal seizures.
Pyridoxine
In infants for whom there is no apparent diagnosis who have ongoing seizures despite Phenobarbital treatment, a trial of Pyridoxine should be considered. Those with an apparent cause such as HIE or sepsis, should proceed to second line agents.
- Pyridoxine – dose may be given IV or orally (if tolerating feeds) – see WoS drug monographs
- Treat for minimum of 3 days
- Ensure cardiac monitoring as risk of apnoeas
- May not see a response for several hours or days
Levetiracetam – option for use as second line (52, 53, 54)
This is being used increasingly frequently as a second line agent for treatment of seizures in the neonate where phenobarbital has not resulted in cessation of seizure activity. The exact mechanism of action of levetiracetam as an anti-seizure medication is poorly understood, but it binds to synaptic vesicle protein SV2a impeding neurotransmitter release and vesicle transport within the neuron.
- Dose – refer to WoS drug monographs for loading and maintenance doses. Treatment is usually commenced intravenously but maintenance doses may be given by the oral route when seizures are controlled and enteral feeds are tolerated.
- Higher doses have been described in the literature, with but should only be used on specialist advice.
- Levetiracetam is well tolerated with a wide therapeutic window and no requirement for monitoring serum levels.
- There are few significant documented side effects in neonates
- Animal models show that levetiracetam does not lead to neuronal apoptosis seen with phenobarbital
- May be preferable to phenytoin due to the variable pharmacokinetics of phenytoin resulting in difficulties establishing dosages.
- Current data is lacking in the use of levetiracetam as a first line agent in treatment of neonatal seizures.
Levetiracetam is not extensively metabolised and it is not metabolised by the liver, so there ae no drug-drug interactions via the cytochrome p450 pathway. It is excreted by the kidneys so dose adjustment may need to be considered in patients with renal impairment.
Phenytoin Sodium – option for use as second Line
This is commonly used as a second line agent when phenobarbital has failed to control seizure activity. Phenytoin affects the sodium channels in the motor cortex of the brain preventing spread of seizure activity to the adjacent cortical areas.
- Loading dose – Refer to WoS drug monographs.
- Administer slowly, over 20 minutes, to avoid cardiac dysrhythmia and CNS depression
- Ensure cardiac monitoring attached
- The blood pressure should also be monitored in the first 1-2 hours after administration. If the baby is not on continuous monitoring then cuff measurements should be performed at 15 minute intervals.
- Has potential for tissue inflammation and necrosis with extravasation
- Maintenance dose not usually required
- If needed refer to pharmacy monograph for doses
Phenytoin is also metabolised by the liver and excreted by the kidneys so there is potential for toxicity if there is hepatic or renal impairment.
Midazolam – Third Line
If seizures are not controlled by phenobarbital and phenytoin addition of midazolam can be trialled. Benzodiazepines also work by facilitating the inhibitory effect of GABA. (15)
Midazolam
- Electively intubate at this point if the infant not already ventilated
- Refer to WoS drug monographs for loading and maintenance doses
- May cause significant respiratory depression and hypotension especially at higher doses.
- Withdrawal may occur if stopped too quickly so infusion should be weaned slowly
Anticonvulsant therapy for patients with no intravenous access
- Midazolam
- By buccal administration
- Refer to WoS drug monographs for dose
OR
- Diazepam
- By rectum (as rectal solution)
- Refer to WoS drug monographs for dose
Withdrawal seizures
If seizures are thought to be caused by opiate withdrawal then first line treatment should be with morphine.
Morphine – IV therapy for acute withdrawal
Dose - If seizures are ongoing, or if the baby has not already been on opiate therapy, consider a loading dose of morphine 50 – 100 micrograms/kg as a short infusion. Note that if the higher dose is used there is a risk of apnoea which may necessitate respiratory support
– maintenance therapy – morphine 10 – 20 micrograms/kg/hr IV infusion
- Please see morphine (oral and IV) monographs and NAS guideline
The prognosis for term and preterm infants has improved in the last decades but seizures remain a feature in those that are at an increased risk of dying or having neurological impairment, developmental delay or epilepsy in later life. (12)This rate is higher when there has been EEG confirmation of seizure activity. (43,44) Prognosis will depend on many factors:
- Underlying cause of seizures
- Seizures secondary to Inborn error of metabolism, cerebral malformations, IVH and global hypoxic ischaemic injury are associated with a poor outcome(12,13,21,23)
- Seizures secondary to stroke, subarachnoid haemorrhages, transient metabolic disturbances, familial cases and ‘fifth day fits’ are all associated with a more favourable outcome(3,12)
- Duration of seizures and response to treatment
- Very frequent recurrent seizures or status epilepticus generally have a very poor outcome(45,46)
- Normal interictal EEG is associated with a good outcome(13)
- Infants with a consistently abnormal background EEG with burst suppression or markedly attenuated background pattern that persists for greater than 12 hours after delivery are associated with a grave outcome. (47)
- Up to 10-20% of infants with neonatal seizures will go to have further seizures in childhood(45)
All infants who have neonatal seizures should be followed up and have neurodevelopmental assessment performed. In most cases (Infants < 30 weeks gestation or infants with HIE will be followed up until 2 (corrected) years of age at the end of which they will have formal developmental assessment). Whether specialities other than neonatology have been involved, follow up will be dependent on the underlying cause and the response to treatment.
A. Normal CFM trace
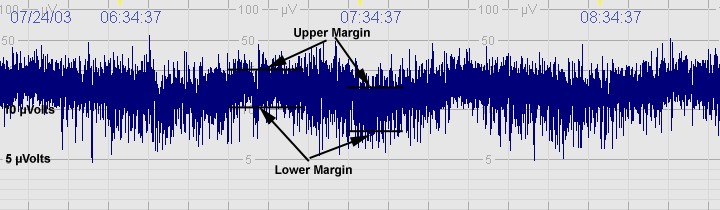
The above CFM tracing shows normal activity with sleep wake cycling seen. The upper margin is >10μvolts with lower margin >5μvolts. Limited variability.
B. Moderately abnormal CFM trace
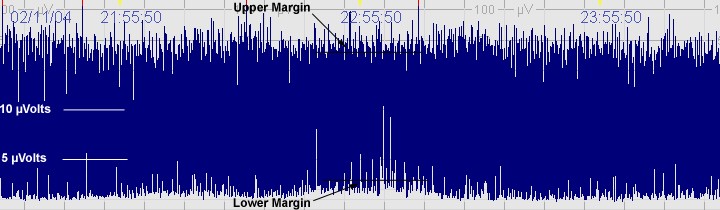
The above CFM tracing shows a moderately abnormal trace with no sleep wake cycling seen. The upper limit is >10μvolts but the lower limits is <5μvolts. There is increased variability.
C. Severely abnormal CFM trace
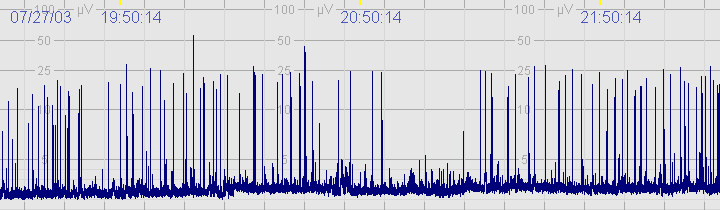 The above CFM trace shows a severely abnormal tracing with no sleep wake cycling seen. The upper limit is <10μvolts and there is very little variability.
The above CFM trace shows a severely abnormal tracing with no sleep wake cycling seen. The upper limit is <10μvolts and there is very little variability.
D. Seizures
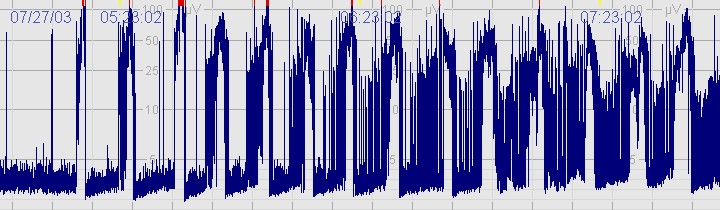 The above CFM tracing shows frequent seizures.
The above CFM tracing shows frequent seizures.
More examples of seizure activity on the CFAM can be found in the following document
Cerebral Function Monitoring – for users of the Olympic CFM 6000
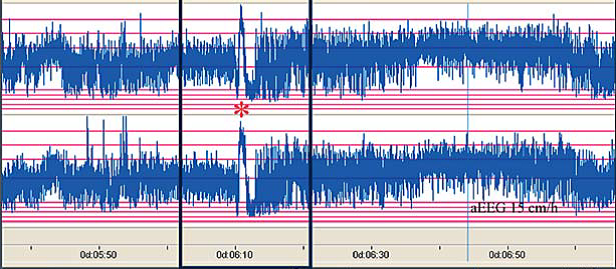
The distinct ictal CFM pattern in newborns with neonatal epilepsy associated with KCNQ2 mutations. (51) Note the sudden rise in upper and lower margin of the aEEG followed by a marked depression of the aEEG amplitude.
(1) van Rooij LGM, Hellström-Westas L, de Vries LS. Treatment of neonatal seizures. Seminars in Fetal and Neonatal Medicine 2013 8;18(4):209-215.
(2) Glass HC, Kan J, Bonifacio SL, Ferriero DM. Neonatal seizures: treatment practices among term and preterm infants. Pediatr Neurol 2012 Feb;46(2):111-115.
(3) Volpe JJ. Neurology of the newborn. 5th ed. Philadelphia: Saunders Elsevier; 2008.
(4) Slaughter LA, Patel AD, Slaughter JL. Pharmacological Treatment of Neonatal Seizures: A Systematic Review. Journal of Child Neurology 2013 March 01;28(3):351-364.
(5) Pisani, Francesco C, Caterina F, Carlo S, Lisa. Neonatal status epilepticus vs recurrent neonatal seizures: Clinical findings and outcome. Neurology 2007 December 4;69(23):2177-2185.
(6) Pressler RM, Mangum B. Newly emerging therapies for neonatal seizures. Seminars in Fetal and Neonatal Medicine 2013 8;18(4):216-223.
(7) Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: A population-based study. Neurology 2007 November 6;69(19):1816-1822.
(8) Glass HC WE. Controversies in Neonatal Seizure Management. Journal of Child Neurology May 2009;24(5):591-599.
(9) Vasudevan C, Levene M. Epidemiology and aetiology of neonatal seizures. Seminars in Fetal and Neonatal Medicine 2013 8;18(4):185-191.
(10) Pressler RM. Chapter 6: Neonatal Seizures. 2015.
(11) Bassan H, Bental Y, Shany E, Berger I, Froom P, Levi L, et al. Neonatal Seizures: Dilemmas in Workup and Management. Pediatr Neurol 2008 6;38(6):415-421.
(12) Uria-Avellanal C, Marlow N, Rennie JM. Outcome following neonatal seizures. Seminars in Fetal and Neonatal Medicine 2013 8;18(4):224-232.
(13) Evans D LM. Neonatal Seizures. Archives of Disease in Childhood. Fetal and Neonatal Edition. 1998;78(1):F70-75.
(14) Booth D, Evans David J. Anticonvulsants for neonates with seizures. Cochrane Database of Systematic Reviews. 2004(3).
(15) Granelli SL, McGrath JM. Neonatal seizures: diagnosis, pharmacologic interventions, and outcomes. J Perinat Neonatal Nurs 2004 Jul-Sep;18(3):275-287.
(16) Mizrahi EM KP. Diagnosis and Management of Neonatal Seizures. Philadelphia: Lippincott-Rave; 1998.
(17) Nardou R, Ferrari DC, Ben-Ari Y. Mechanisms and effects of seizures in the immature brain. Seminars in Fetal and Neonatal Medicine 2013 8;18(4):175-184.
(18) Levene M. Recognition and management of neonatal seizures. Paediatrics and Child Health 2008 4;18(4):178-182.
(19) Sivaswamy L. Approach to neonatal seizures. Clin Pediatr 2012 May;51(5):415-425.
(20) Zupanc ML. Neonatal Seizures. Pediatric Clinics of North America 2004;51:961-978.
(21) Glass,H.C, Glidden D, Jeremy RJ, Barkovich AJ, Ferreira DM, MIller SP. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic ischemic brain injury. The Journal of Pediatrics 2009;155(3):318-323.
(22) Lynch J. Epidemiology and Classification of Perinatal Stroke. Seminars in Fetal and Neonatal Medicine 2009;14:245-249.
(23) Silverstein FS JF. Neonatal Seizures. Ann Neurol 2007;62:112-120.
(24) Laine K, Heikkinen T, Ekblad U, Kero P. Effects of Exposure to Selective Serotonin Reuptake Inhibitors During Pregnancy on Serotonergic Symptoms in Newborns and Cord Blood Monoamine and Prolactin Concentrations. Arch Gen Psychiatry 2003;60(7):720-726.
(25) Haddad PM, Pal BR, Clarke P, Wieck A, Sridhiran S. Neonatal symptoms following maternal paroxetine treatment: Serotonin toxicity or paroxetine discontinuation syndrome? Journal of Psychopharmacology 2005 September 01;19(5):554-557.
(26) Sanz EJ, De-las-Cuevas C, Kiuru A, Bate A, Edwards R. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. The Lancet 2005 2/5–11;365(9458):482-487.
(27) Sheth RD, hobbs GR, Mullett M. Neonatal Seizures: incidence, onset and aetiology by gestational age. J Perinatol 1999;19:40-43.
(28) Dehan M, Gabilan JC, Navelet Y, D'Allest Am. Fifth day fits. Archives of Disease in Childhood 1982;57:400-401.
(29) Rennie JM, Boylan GB. Neonatal seizures and their treatment. Curr Opin Neurol 2003 Apr;16(2):177-181.
(30) Helen Cross J. Differential diagnosis of epileptic seizures in infancy including the neonatal period. Seminars in Fetal and Neonatal Medicine 2013 8;18(4):192-195.
(31) Hallberg B, Blennow M. Investigations for neonatal seizures. Seminars in Fetal and Neonatal Medicine 2013 8;18(4):196-201.
(32) Jensen FE. Neonatal Seizures: An Update on Mechanisms and Management. Clin Perinatol 2009;36(4):881.
(33) Boylan GB, Stevenson NJ, Vanhatalo S. Monitoring neonatal seizures. Seminars in Fetal and Neonatal Medicine 2013 8;18(4):202-208.
(34) Shellhaas RA, Barks AK. Impact of amplitude-integrated electroencephalograms on clinical care for neonates with seizures. Pediatr Neurol 2012 Jan;46(1):32-35.
(35) Painter MJ, Scher MS, Stein AD, Armatti S, Wang Z, Gardiner JC, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med 1999 Aug 12;341(7):485-489.
(36) Castro Conde JR, Hernandez Borges AA, Domenech Martinez E, Gonzalez Campo C, Perera Soler R. Midazolam in neonatal seizures with no response to phenobarbital. Neurology 2005 Mar 8;64(5):876-879.
(37) Sirsi D et al. Successful Management of Refractory Neonatal Seizures with Midazolam. Journal of Child Neurology 2008;23(6):706-709.
(38) Sheth RD, Buckley DJ, Gutierrez AR et al. Midazolam in the treatment of refractory neonatal seizures. Clinical Neuropharmacology 1996;19:165-1.
(39) van den Broek MP, Huitema AD, van Hasselt JG, Groenendaal F, Toet MC, Egberts TC, et al. Lidocaine (lignocaine) dosing regimen based upon a population pharmacokinetic model for preterm and term neonates with seizures. Clin Pharmacokinet 2011 Jul;50(7):461-469.
(40) Lundqvist M, Ågren J, Hellström-Westas L, Flink R, Wickström R. Efficacy and safety of lidocaine for treatment of neonatal seizures. Acta Paediatrica 2013;102(9):863-867.
(41) Boylan GB, Rennie JM, Pressler RM, et al. Phenobarbitone, neonatal seizures and video-EEG. Archives of Disease in Childhood 2002;86:165-170.
(42) van Rooij LG, van den Broek MP, Rademaker CM, de Vries LS. Clinical management of seizures in newborns : diagnosis and treatment. Paediatr Drugs 2013 Feb;15(1):9-18.
(43) McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurologu 2000;55:506-513.
(44) West CR, Harding JE, Williams CE. Cot-side electroencephaliography for outcome prediction in preterm infants: observational study. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2011;96:F108-F112.
(45) Tekgul H, Gauvreau K, Soul J, Murphy L, Robertson R, Stewart J, et al. The Current Etiologic Profile and Neurodevelopmental Outcome of Seizures in Term Newborn Infants. Pediatrics 2006 April 01;117(4):1270-1280.
(46) van Rooij LGM, de Vries LS, Handryastuti S, Hawani D, Groenendaal F, van Huffelen AC, et al. Neurodevelopmental Outcome in Term Infants With Status Epilepticus Detected With Amplitude-Integrated Electroencephalography. Pediatrics 2007 August 01;120(2):e354-e363.
(47) Pressler RM, Boylan GB, Morton M et al. Early serial EEG in hypoxic ischaemic encephalopathy. Clinical Neurophysiology 2001;112:31-37.
(48) Pressler RM, Cilio MR, Mizrahi EM, Moshes SL, Nunes ML, Plouin P, Vanhatalos S, Yozawitz E, Zuberi S. The ILAE Classification of Seizures and the Epilepsies: Modification for Seizures in the Neonate. Proposal from the ILAE Task Force on Neontal Seizures. ILAE website, 2018.
(49) Gonsales MC, Montenegro MA, Soler VC, Coan AC, Guerreiro MM, Lopes-Cendes I. Recent developments in the genetics of childhood epileptic encephalopathies: impact in clinical practice. B Arq Neuropsiquiarti 2015:1-13
(50) Cornet MC, Sands TT, Cilio MR. Neonatal epilepsies: Clinical management. Seminars in Fetal & Neonatal Medicine 2018;23:204-212
(51) Vilan A, Ribeiro JM, Striano P, Weckhuysen S, Weeke LC, Brilstra E, de Vries LS, Cilio MR. A distinctive amplitude-integrated electroencephalography pattern in newborns with neonatal epilepsy associated with KCNQ2 mutations. Neonatology 2017;112:387-393
(52) Mruk AL, Garlitz KL, Leung NR. Levetiracetam in neonatal siezures: a review. Journal of Paediatric Pharmacology 2015;20(2):76-89
(53) Ahmad KAA, Desai JJ, Bennett MM, Ahhad SF, Ng Y-T, Clark RH, Tolia VN. Changing anti-epileptic drug use for seizures in US neonatal intensive care units from 2005 to 2014. Journal of Perinatology 2017;37:296-300
(54) Han JY, Moon CJ, Youn YA, Sung IK, Lee IG. Efficacy of levetiracetam for neonatal seizures in preterm infants. BMC Pediatrics 2018;18:131
(55) Sharpe C, Reiner GE, Davis SL, et al. Levetiracetam Versus Phenobarbital for Neonatal Seizures: A Randomized Controlled Trial. Pediatrics. 2020;145(6):e20193182).
Last reviewed: 24 October 2022
Next review: 01 October 2025
Author(s): Last updated by Dr Carolyn Abernethy – Consultant Neonatologist – Princess Royal Maternity – Oct 22
Co-Author(s): Previously updated by Dr Karen Walsh - ST7 Neonatal Grid Trainee - WoS Paediatric rotation. Other Professionals consulted: Prof Sameer Zuberi – Consultant Paediatric Neurologist, Royal Hospital for Sick Children, Glasgow; Peter Mulholland – Neonatal Pharmacist – Southern General Hospital, Glasgow; June Grant – Neonatal Pharmacist – Princess Royal Maternity, Glasgow
Approved By: WoS Neonatology MCN

