Major haemorrhage protocol
Objectives
- Allow rapid and appropriate response to major haemorrhage.
- Open channel of communication between clinical area and Blood Bank.
- Provide quick and effective delivery of blood components for patients with a major haemorrhage.
Subjective
Clinical concern that patient is experiencing bleeding problems requiring multiple transfusion support – clinician discretion
Objective
- 20% blood loss in < 1 hour.
- 50% blood volume loss in < 3 hours. (Infant blood volume 90ml/kg, older child 80 ml/kg).
An experienced clinician determines that the patient fulfils one of the above criteria
How to activate Major Haemorrhage Protocol
Phone 2222 and say “Major Haemorrhage, Children's Hospital” stating location of patient (i.e. ward / department).
Blood Bank will call the ward.
Porter will go to the Blood Bank.
General response
- Control bleeding
- Venous access
- Avoid hypothermia - warm fluids
- Take appropriate blood tests and send urgently to appropriate Laboratory
Immediate blood tests
- FBC
- Crossmatch
- Coagulation screen (including Fibrinogen)
- Biochemistry (including Calcium
- Blood gases (if appropriate).
Information required by Blood Bank
- Urgency of the situation
- Patient details:
Minimum data set:
- Conscious patient – forename, surname; DOB; gender; CHI number.
- Unconscious/unidentified patient – minimum of gender and CHI number. - Whether cross match has been sen
- Location of Patient
- Contact number – need to establish clear lines of communication
- Patient diagnosis
Nominate one person to liaise with Blood Bank
Blood component availability
Factor in time for samples to reach Blood Bank and any blood product to be delivered from Blood Bank.
- Immediate – Group O Negative blood
(4 units available in Paediatric theatres fridge) - 15 minutes – group specific blood (ABO + RhD grouping)
- 35 minutes – fully cross-matched blood.
- Platelets – Platelets supplies are limited so these may be delayed.
- Fresh Frozen Plasma (FFP) and Cryoprecipitate – allow up to 20 minutes for thawing (plus delivery time)
Massive haemorrhage
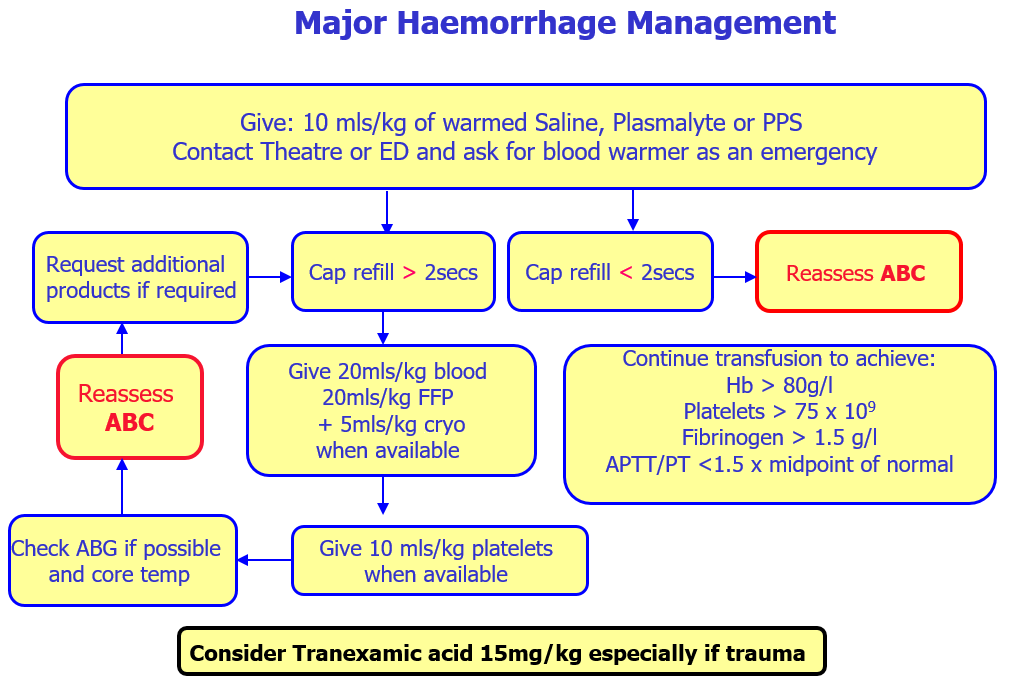
Access nearest available Group O Negative units
Give 20ml/kg blood when available, 20ml/kg FFP and 5ml/kg cryo should be given.
This does not require coagulation screen results.
Give 10ml/kg platelets when available.
Continue transfusion to achieve:
Hb > 80g/l
Platelets > 75 x 109
Fibrinogen > 1.5 g/l (if not, transfuse 5ml/kg of cryo)
APTT/PT <1.5 x midpoint of normal (if not, transfuse 20ml/kg of FFP)
Additional interventions
Consider tranexamic acid 15mg/kg infused over 15 minutes followed by 2mg/kg infusion, for at least 8 hours or until bleeding stops. This treatment can be started within 3 hours of haemorrahage.
Novo 7, Recombinant factor VIIa (rFVIIa) – although current recommendation is that Novo 7 is not for use outside licensed indication, consideration should be given to its use where treatment options are limited and patient is exsanguinating. Please contact Consultant Haematologist.
Management of adverse complications
The following complications should be anticipated and managed appropriately in patients receiving multiple units of blood components.
Hypothermia – monitor temperature, keep patient warm. Consider active warming.
Hyperkalaemia – monitor potassium, initiate local protocol for treatment of any hyperkalaemia (glucose + insulin + bicarbonate).
Acidosis – monitor patient closely, take corrective action.
Hypocalcaemia – monitor calcium levels, correct as appropriate.
Management of Warfarin reversal
Stop warfarin
Give Vitamin K (30mcg/Kg) IV, consider higher doses if INR >8
Clotting factor replacement is required when there is active bleeding. 4-Factor Prothrombin Complex Concentrate (PCC / trade name “Beriplex”) replacement therapy gives superior clotting factor replacement compared to FFP.
- Dosage is calculated based on the current INR and patient’s weight. (see table)
INR
Approximate Dose
2.0 - 3.9
1ml/kg = 25iu/kg
4.0 - 6.0
1.4ml/kg = 35iu/kg
>6.0
2 ml/kg = 50iu/kg
- Repeat the INR following Beriplex.
- Further doses of Beriplex or Vitamin K may be required.
To order Beriplex discuss with on call Haematologist and request via TrakCare.
It is essential that Blood Bank is informed whenever the clinical emergency has ended to minimise wastage of blood components. This is the responsibility of the clinical lead / nurse coordinator.
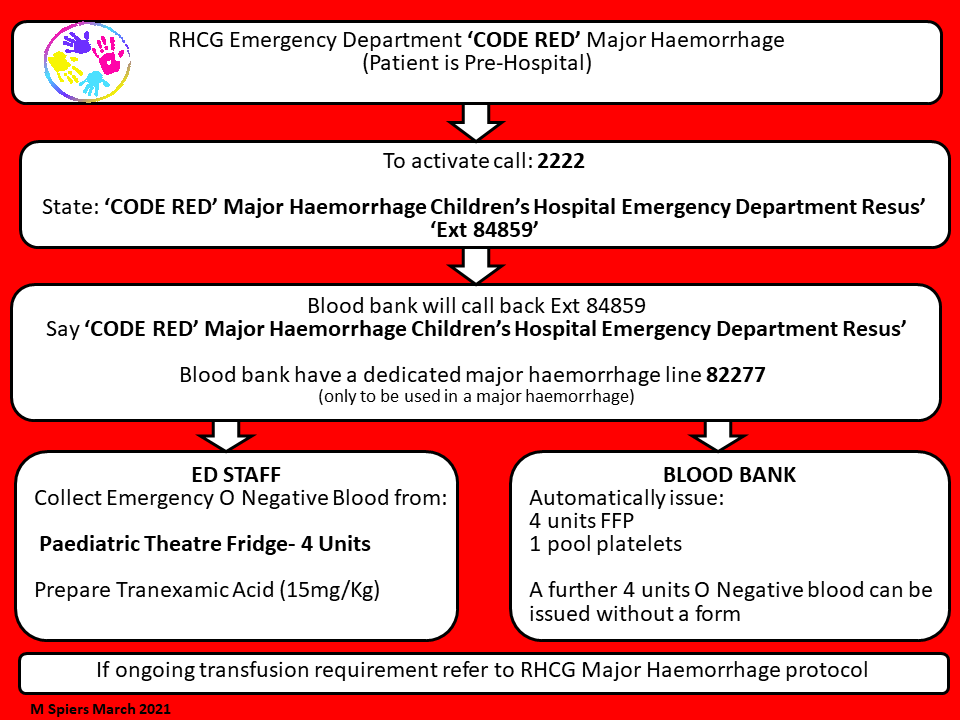
- In the event of a pre-hospital ‘code red’ the MH protocol should be triggered by calling 2222 and stating ‘code red major haemorrhage’ and stating location.
- Activation should result in immediate provision of 4 units PRCs and 4 units FFP (pre-thawed) as a pack regardless of age and weight, and a designated porter to transport specimens and blood products to and from the labs.
- The first pack can be issued without forms.
- O neg blood can be obtained directly from RHC theatres fridge if needed but blood bank should be informed it has been removed.
- Following triggering of the major haemorrhage protocol - blood bank should phone ED to confirm further product requirements. If not instigated by blood bank then ED team to contact blood bank. Named contact person and direct dial number should be obtained for blood bank and ED contact person. Further product requirements and progress of product requests should be communicated between these two representatives.
- Products should be given as per protocol (20ml/Kg PRC/ 20ml/Kg FFP/ 10mls/Kg PLT and 5ml/Kg cryo) - aiming for 1:1:0.5 (PRC/FFP/PLT) ratio. Cryoprecipitate will always take longer to arrive than other products.
Activation of the major haemorrhage response will be audited by the local Hospital Transfusion Committee so that defects in the process can be identified, rectified, and lessons learned fed back to all staff involved in the major haemorrhage response.
NOTES:
- Assess weight of patient
- Platelet availability: a limited stock of platelets are held on-site
- Always remember 'thaw time' for Cryo and additional FFP can be up to 20 mins
- The EZIO drill is a specifically designed intraosseus drill for fluid and blood administration, with sizes appropriate for all ages. It is available in ED and Theatre 6 (84358)
- The laboratory cannot process a marrow sample
- Blood warmer can be obtained from Theatre (ext 84358)
- Blood Bank Major Haemorrhage (ext 82277)
- MLA Blood Runner page 17262
- REMEMBER COMMUNICATION LEAD MUST NOTIFY BLOOD BANK TO 'STAND DOWN' WHEN APPROPRIATE

Team alerted by 2222 Major Haemorrhage call to switchboard:
- Blood Bank
- Porter
- Resuscitation Team
- Haematologist on call
- Hospital Co-ordinator
Contact
- Emergency page will alert the team to clinical area involved in the major haemorrhage
- Blood Bank will call the clinical area to identify the patient and obtain details of the haemorrhage
- Porter will go to the Blood Bank
- The Senior Nurse will act as the link between the clinical area and Blood Bank.
4 units of emergency use Group O negative red cells are available in paediatric theatre fridge (barcode access).
- Porters will collect emergency Group O-negative blood without a collection form.
Contact details for Blood Bank
Ext number 803930
Page 8039 or via switchboard
Information required by Blood Bank:
- Urgency of the situation
- Patient details:
Minimum data set:
- Conscious patient – forename, surname; DOB; gender; CHI.
- Unconscious/unidentified patient – minimum of gender and CHI. - Location of Patient
- Contact number – need to establish clear lines of communication.
- Patient diagnosis
- Confirm which samples have been sent
Nominate one person to liaise with Blood Bank
British Committee for Standards in Haematology (2011) Guidelines on oral anticoagulant therapy.
British Journal of Haematology. Royal College of Paediatrics and Child Health (2012) Evidence Statement: Major Trauma and the use of tranexamic acid in children.
Last reviewed: 01 September 2021
Next review: 30 September 2023
Author(s): P Bolton, E Harrison
Version: 5
Approved By: Clinical Effectiveness