Clostridioides (Clostridium) Difficile Infection (CDI) in children: diagnosis and management
exp date isn't null, but text field is
In adults Clostridioides difficile (C.difficile) is a common cause of hospital acquired diarrhoea. In children the role of C.difficile is less well understood and in the majority likely represents gut colonisation. Recent studies however have shown that C.difficile is an emerging pathogen in the paediatric setting particularly in patient with underlying haemato-oncology and gastrointestinal disorders. Other risk factors include recent antibiotics, proton pump inhibitors and prolonged hospitalisation.
Health Protection Scotland recommend testing for CDI in all symptomatic children aged 3 years or older. Testing of diarrhoeal stools from children under the age of 3 years should be at the clinicians request only.
In all cases, testing results must be carefully evaluated against the clinical background of the patient.
In GGC diagnostic labs test diarrhoeal samples for CDI in two stages. Firstly all diarrhoeal stool samples are tested using a sensitive screening test – GDH (glutamate dehydrogenase).
If the GDH test is negative the stool sample is reported as negative for CDI
If the GDH test is positive the lab proceeds to the second stage of testing which is toxin detection.
The interpretation of results is as follows;
|
Result |
Interpretation |
|
GDH negative |
No CDI or colonisation |
|
GDH positive, toxin negative |
Colonised with C.difficile. Unlikely to require treatment. Send repeat samples if symptoms persist |
|
GDH positive, toxin positive |
CDI possible if case definition met |
GDH and toxin positive sample with diarrhoea and one or more of the following
- Significant co morbidity – Gastrointestinal disease, haemato-oncological condition
- Antibiotic use in the last 4 weeks
- Severe GI disease with bloody diarrhoea and an unlikely alternative diagnosis.
Further investigation:
- Send FBC , CRP and U+Es
- Request imaging ( abdominal X-ray) if toxic megacolon or ileus is suspected
- Test for clearance is not required
Patients who meet the above case definition should undergo daily severity scoring as below and be managed accordingly
|
Criteria |
Point |
|
Diarrhoea > 5 times/day |
1 |
|
Abdominal pain and discomfort |
1 |
|
Rising white cell count |
1 |
|
Raised CRP |
1 |
|
Pyrexia > 38oC |
1 |
|
Evidence of pseudomembranous colitis |
2 |
|
Intensive care requirement |
2 |
Score:
1-2 = Mild disease
3-4 = Moderate disease
≥5 = Severe disease
- Review all antibiotics. Where possible discontinue or switch to less C.diffogenic agents.
- PPI /H2 antagonist use should be reviewed and if possible discontinued
| Disease severity | Severity score (points) | Treatment |
|---|---|---|
| Mild | 1-2 | May not require treatment. Consider pharmacological treatment if symptoms persist |
| Moderate / severe | >3 | Treat as per table below. Re-assess daily. For treatment failure seek urgent specialist advice |
| Life threatening | Seek urgent specialist advice Consider surgical intervention if evidence of caecal dilatation on imaging. |
|
Treatment
All drug doses as per BNF for Children or Summary of Product Characteristics (SmPC)
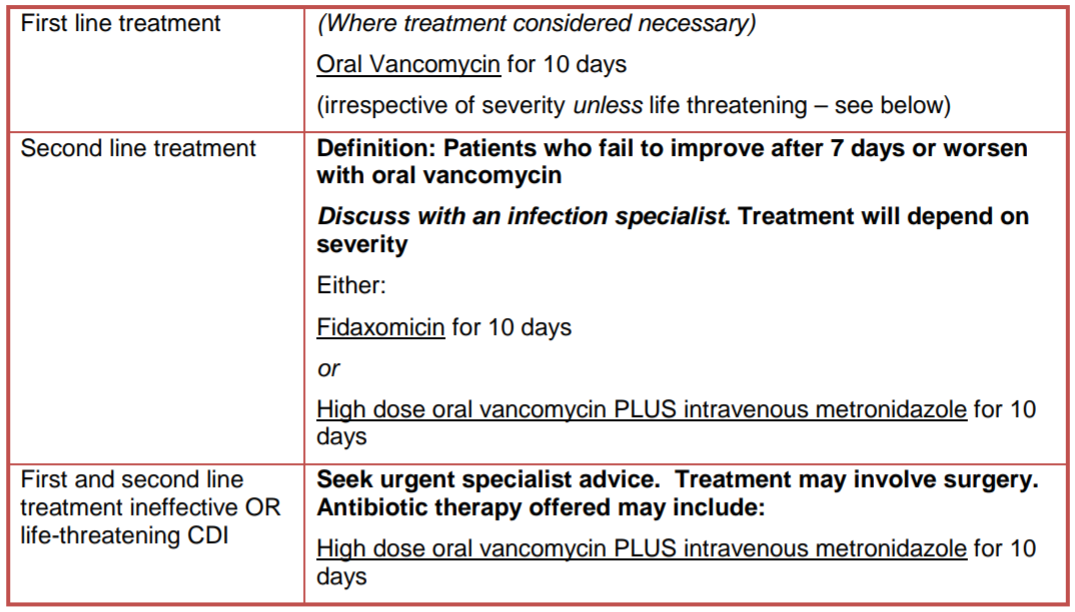
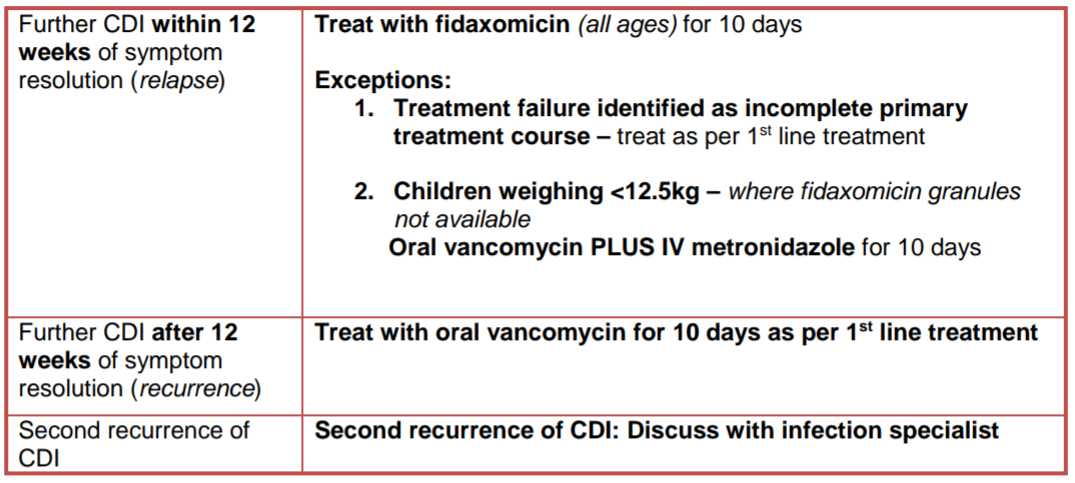
For first recurrence of CDI please follow the management advice below. If relapse/recurrence is severe/life threatening seek urgent specialist advice as above. For second recurrence discuss with an infection specialist.
Please refer to the NHSGGC CDI infection control policy
Management in Primary Care:
Metronidazole may be prescribed in community if delays in supply of oral vancomycin would result in delayed initiation of treatment. Metronidazole should be substituted with oral vancomycin as soon as availability is resolved to complete a total of 10 days treatment. For patients who cannot swallow vancomycin capsules, fidaxomicin should be considered the first line option in community setting.
Fidaxomicin:
Fidaxomicin tablets are licensed in children weighing at least 12.5kg. Fidaxomicin granules for oral suspension (40mg/ml) became available in the UK in May 2022. For children weighing less than 12.5kg consider oral vancomycin PLUS IV metronidazole if granules unavailable and/or seek specialist advice.
Vancomycin:
Vancomycin injection can be used via the oral route in the hospital setting where capsules are not available or where the calculated dose cannot be administered using available capsule strengths. Guidance on administration is available below:
To administer vancomycin via an NGT;
- Use vancomycin injection, 500mg vial.
- Reconstitute a 500mg vial with 10ml water for injection to give a 50mg/ml solution.
- Calculate the dose and withdraw the required amount from the vial. Discard the remainder of the vial.
- Flush the enteral feeding tube with 15mls sterile water prior to administration. The dose can be further diluted with water for injection if required.
- Administer the dose and flush with a further 15ml sterile water.
- Recommended protocol for testing for Clostridium difficile and subsequent culture. Health Protection Scotland , October 2016
- Pai S et al. Five years experience of Clostridium difficile infection in children at a UK tertiary hospital: proposed criteria for diagnosis and management. PLOS 2012;71-6
- ‘Guidance for the prevention and control of clostridium difficile infection (CDI) in health and social care settings in Scotland’; Scottish Health Protection Network; Guidance No 6. 2017 edition
- ‘Updated advice on antimicrobial management of Clostridioides Difficile (C.diff) Infection (CDI) in childre’; Scottish Antimicrobial Prescribing
- ‘Clostridioides difficile infection: antimicrobial prescribing’; NG 199; NICE; 23rd July 2021
- ‘Safety and Efficacy of fidaxomicin and vancomycin in children and adolescents with clostridioides (clostridium) difficile infection: A Phase 3, multicenter, randomised single-blind clinical trial (SUNSHINE); Clinical Infectious Diseases 2020:71; (15th November 2020)
Last reviewed: 24 August 2022
Next review: 01 August 2025
Author(s): Susan Kafka
Version: 3
Co-Author(s): Guideline development group: Dr T Inkster, Consultant Microbiologist; Dr A Deshpande, Consultant Microbiologist; Dr C Doherty, Paediatric ID Consultant; Dr R Hague, Paediatric ID Consultant; Kathleen Harvey-Wood, Clinical Scientist, Microbiology; Gillian Bowskill , Lead Nurse, Infection Prevention and Control; Susan Kafka, Advanced Pharmacist, Paediatric Antimicrobials and Medicine.
Approved By: Antimicrobial Utilisation Committee

