Management of Atypical Genitalia & Suspected DSD in the Neonate
exp date isn't null, but text field is
Objectives
This document is applicable to all medical, nursing and midwifery staff caring for the newborn in hospital or the community. The main focus of this document is on the management of the child with suspected disorder of sex development (DSD).
Disorders of sex development (DSD) are relatively rare conditions with diverse pathophysiology. These conditions are very heterogeneous and can present in a variety of different ways, most commonly with atypical genitalia in the newborn period or as a disorder of puberty in adolescence (Appendix 1). These clinical situations can often be difficult to manage, particularly when sex assignment is difficult. The needs of each individual patient must be fully considered, along with the needs of their families and cultural practices. The multidisciplinary team should be involved from an early stage. Families should be provided with as much information as possible (Appendix 3).
Examine each testis to determine if they are palpable/impalpable and descended (within the scrotum) or undescended (outwith the scrotum).
|
Testis 1 |
Testis 2 |
Next step |
|
Undescended but palpable |
Descended and palpable |
Send letter to GP and ask to re-check at 6 week check and refer to Paediatric surgery if warranted |
|
Undescended and impalpable |
Descended and palpable |
Send letter to GP and ask to re-check at 6 week check and refer to Paediatric surgery if warranted |
|
Undescended but palpable/seen on USS |
Undescended but palpable/seen on USS |
Treat as bilateral undescended testes below |
|
Undescended and impalpable |
Undescended and impalpable |
Treat as bilateral undescended testes below |
If a patient has hypospadias that is not penoscrotal or perineal and no other features of concern are present, no immediate action is required. Advise the parents against circumcision until after surgical review and refer to the Urology Team at RHC. A good urinary stream should be observed prior to discharge.
A child with a suspected DSD may present with one or more of these features:
- a bifid scrotum
- bilateral undescended testes
- clitoromegaly (phallus >0.8mm in an apparent female infant)
- micropenis (phallus <2cm stretched penile length in an apparent male infant)
- proximal (penoscrotal or perineal) hypospadias
- distal or midshaft hypospadias in combination with any of the above features
If any of these features are detected at delivery and there is uncertainty about the sex of the baby, explain to the parents that it is not possible to tell whether their infant is a girl or a boy. Do not guess the sex, or even voice your suspicions as this can be unhelpful to the parents - it is extremely difficult to ‘change’ the sex of a baby in the light of results if the parents have started to adjust to the baby being a particular sex, and may have told friends or relatives this sex.
Advise the parents not to name the baby, and to think carefully about who they tell that the baby has been born, as this may be an issue that they wish to remain confidential until a sex has been assigned. A single room would allow more frequent private discussions.
Registration of the birth must be delayed until the sex has been assigned. The baby should be referred to as ‘your baby’ or ‘the baby’ but not ‘it’, ‘he’ or ‘she’ and a white cot card (not blue or pink)) can be used.
Clinical psychology support should be offered to the parents of every newborn where sex assignment is delayed. Please contact the Clinical Psychology Service at RHC, Glasgow on 0141 451 6574.
In the event that the above features are picked up late, i.e at neonatal discharge check when sex may have already been assigned, the neonatal consultant on-call should be informed on the day so that the concerns regarding the genitalia can be conveyed to parents. Advise the parents that the sex of the baby is uncertain, and further assessment will need to be carried out over the next few days.
- The neonatal consultant on call should be informed about the baby.
- History and examination should be carried out by an experienced clinician (Appendix 2).
- The patient should be discussed during working hours with the Endocrine Registrar at the Royal Hospital for Children (RHC), Glasgow, who can be contacted on 0141 451 5799 (85799 while in hospital), and who will liaise with the Endocrinology Consultant on-call. They will be asked to document any discussions on Clinical Portal even if they do not attend to see the child.
|
Sample |
Test |
Why do you do it? |
How do you do it? |
Where does it go? |
|
Blood |
QF-PCR |
This test will identify the presence or absence of the Y chromosome which will help with sex assignment |
Send EDTA sample. Contact the Duty Geneticist, Molecular Genetics lab (59310) to say the request is urgent. Although the lab has a 3 day turnaround time for this test, a QF-PCR will usually be available within 24 hours, if received before 3pm. Important: |
Queen Elizabeth University Hospital Genetics laboratories
|
|
Blood |
Microarray |
This test will confirm the presence or absence of the Y chromosome identified by QF-PCR and provide further information on chromosomal variations |
Contact the Duty Geneticist, by email: geneticlabs@ggc.scot.nhs.uk Molecular Genetics 64057 Important: |
Queen Elizabeth University Hospital Genetics laboratories
|
|
Imaging |
USS Pelvis
|
This test can identify the presence or absence of Mullerian structures and help with sex assignment |
Discuss with the duty radiologist at local hospital for an urgent scan |
If a transfer is planned to RHC then liaise with Endocrine Team |
Second Line Tests - These tests should be performed after discussion with RHC Endocrine Team and will depend on the clinical presentation
|
Specimen |
Investigations |
How do you do it? |
Where does it go? |
|
Blood |
UE, glucose and cortisol |
Lithium heparin sample after day 3. |
Local laboratory |
|
Blood |
17-OH progesterone |
Lithium heparin sample. Ideally take >36 hours after birth to allow the postnatal surge to subside. Contact Duty Biochemist (89060) to inform of sample sent |
Local Lab who will send to GRI Biochemistry labs |
|
Blood |
AMH, FSH, LH, testosterone |
Lithium heparin samples (2ml)
|
Queen Elizabeth University Hospital Biochemistry labs |
|
Blood |
Store sample for ACTH, DHAS, androstenedione |
EDTA (minimum 2ml)
|
Queen Elizabeth University Hospital Biochemistry labs |
|
Urine |
Steroid profile |
15-20ml non-sterile collection in universal container, ideally with collection starting on/after day 3. Give as much clinical details as possible to aid interpretation of results |
Local Lab who will send to GRI Biochemistry labs |
Further investigations to identify the cause of DSD are discussed in Appendix 3 but should not be ordered without discussion with the endocrine team. These may include:-
- hCG stimulation test, this will normally be carried out at RHC
- DNA analysis
- Imaging by Laparoscopy/Genitogram/Genitoscopy
The process of bringing together some of the initial information usually takes about 5 working days.
All abnormal sex chromosome results should be flagged to the on-call endocrine consultant once identified.
In some cases, however, where sex assignment is difficult and/or the chromosome result is complicated, laparoscopy may be required, and the process may take longer and often up to 2 weeks. It is important to try to avoid burdening the parents with too much information during the process, as this may give conflicting messages about the sex before final conclusions are reached. This is especially true where some investigations such as ultrasound may be difficult to interpret in a newborn. Although it is important to explain why tests are being done, it is probably best to try to bring things together for the parents once all the information is available. To this end it is helpful to have a surgical opinion early, as this gives a view of the long-term anatomical possibilities. Results will be explained to the family by a consultant member of the DSD team.
Depending on the case, ongoing management may require the help of several members of the DSD MDT. The extent and frequency of this will be decided by the DSD team prior to discharge in the more complex cases. As a default, all cases of suspected DSD should be reviewed at the second Monday of the month clinic held by Prof Ahmed at RHC which is also attended by Emily Fraser from Clinical Psychology and Dr Ruth McGowan from Clinical Genetics.
Biology of DSD
Primordial germ cells migrate to the genital ridge from the yolk sac at approximately 6 weeks of gestation in the human embryo. Wilms Tumour 1 (WT1) and Steroidogenic Factor 1 (SF1) genes result in the development of bipotential gonads from these cells.
Usually, if a Y chromosome is present, the development of Mullerian structures is inhibited by the production of a glycoprotein called Anti-Mullerian Hormone (AMH). With the production of testosterone by the Leydig cells, the mesonephric (Wolffian) duct increases in size and differentiates into the epididymis, vas deferens and prostate. 5-dihydrotestosterone (DHT) is produced by the conversion of testosterone by the enzyme 5α-reductase, resulting in the development of male external genitalia and testicular descent. If there is no Y chromosome present, the Mullerian structures usually develop into female internal genitalia and ovaries develop. The absence of DHT also results in the development of female external genitalia. There are many factors involved in the differentiation of the sex organs into male or female and there is potential for a disruption of this process at multiple different stages. The clinical phenotype will therefore depend on the nature of disruption.
Classification of DSD
There are three broad groups: sex chromosome DSD, 46,XY DSD and 46,XX DSD.
Sex chromosome DSD
Sex chromosome DSD includes conditions such as 47,XXY (Klinefelter syndrome and variants), 45,X (Turner syndrome and variants), 45,X/46,XY (mixed gonadal dysgenesis) and 46,XX/46,XY (chimerism). These are often diagnosed antenatally with confirmation of the diagnosis after birth. Antenatal diagnosis allows for focussed evaluation of the other complications associated with these disorders, for example, cardiac anomalies in Turner syndrome. It also provides the opportunity to offer counselling to families prior to the birth.
46,XY DSD
46,XY DSD has three broad categories: disorders of gonadal (testicular) development, disorders in androgen synthesis or action and other causes, including hypogonadotrophic hypogonadism, cryptorchidism and isolated hypospadias. They are a heterogeneous group of disorders, where the phenotype is consistent with reduced male sex hormone action.
46, XX DSD
46, XX DSD encompasses disorders of gonadal (ovarian) development, such as gonadal dysgenesis and disorders secondary to androgen excess. Most commonly, the high levels of androgens responsible for virilisation in 46,XX DSD patients are secondary to production by the foetal adrenal glands and amongst them 21 hydroxylase deficiency CAH is the most common disorder. Androgen excess during pregnancy may be endogenous (secondary to an adrenal adenoma, dermoid cyst, Sertoli-Leydig tumour, sex cord stromal tumour or metastatic carcinoma) or exogenous (secondary to danazol, progestins or potassium sparing diuretics). Exogenous steroids taken during pregnancy can also cause posterior fusion of the labia, clitoral enlargement and increased degrees of androgenisation. Where clinically possible, mothers should be advised of the potential risks associated with taking medications which increase androgen production during pregnancy and an alternative sought.
A focussed clinical history should be taken from the parents, addressing the following issues:
- Parental consanguinity, history of salt-losing, unexplained infant deaths or DSD in relatives. These elements may indicate autosomal recessive genetic disorders associated with disturbed steroidogenesis.
- Maternal ingestion of drugs or exposure to specific environmental factors capable of inhibiting virilisation of the foetus during the pregnancy.
- Whether the pregnancy was planned - Some of the progestogen-containing drugs used for assisted-conception techniques are associated with a higher likelihood of male offspring with genital anomalies.
- In cases where parents have had some prenatal advice and discussion, it is useful to have access to these previous discussions and to seek parents’ recollection of these discussions.
- Results of prenatal tests if available.
- Social history with an enquiry about the family’s social network.
In particular the clinician should look out for:
- Any dysmorphic features, in particular midline defects (suggesting abnormalities of the hypothalamic-pituitary axis).
- The state of hydration and blood pressure (daily measurement until formal diagnosis)
- Jaundice (associated with hypopituitarism)
- Urine dip for protein (associated with renal anomalies)
- Pre feed blood glucose – commence hypoglycaemia screening protocol (see neonatal guidelines)
- Examination of the external genitalia
- Inspection - Is there more than expected pigmentation of the genitalia with a genital anomaly? If so the baby may have congenital adrenal hyperplasia (CAH)
- Palpation for gonads or swellings in labioscrotal fold or inguinal region.
If there are palpable gonads, the baby is probably a male (but don’t share this opinion with parents) - There are various scoring systems available to assess the external genitalia. We recommend the use of the External Masculinising Score initially, shown below. This is useful to tell the Endocrine team when referring the patient.
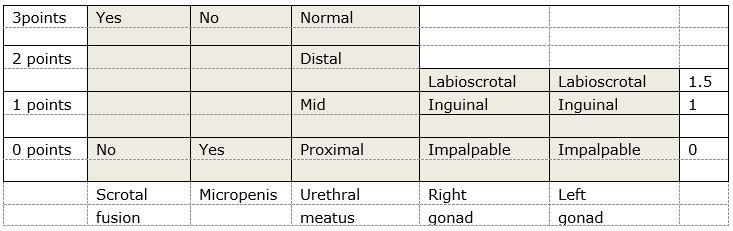
Websites
DSD Families - An information and support resource for families with children, teens and young adults who have a DSD
Information Leaflets
The following information leaflets are available on the Scottish DSD network website at this URL: https://www.sdsd.scot.nhs.uk/families/
- What is a DSD?
- DSD Families – When your baby is born with genitals that look different… The first days.
- The Story of Sex Development
- Nursery and Your Son
- For parents of children with peno-scrotal/ proximal hypospadias and differences of sex development
- Congenital Adrenal Hyperplasia (CAH) Information Sheet
- Information about Genetic Testing Sheet
- Genetic Testing Information for Families
- Hypospadias for parents
- Mayer-Rokitansky-Küster-Hauser (MRKH) Information Sheet
- Undescended Testes Information Sheet
- Vaginal Dilation Information Sheet
- Glossary of Terms
Current Version Authors
- Angela Lucas-Herald, Clinical Lecturer, RHC, Glasgow
- Faisal Ahmed, Consultant Endocrinologist, RHC, Glasgow
- Emily Fraser, Psychologist, RHC, Glasgow
- Ruth McGowan, Consultant Geneticist, QEUH, Glasgow
- Jane McNeilly, Consultant Biochemist, QEUH, Glasgow
- Stuart O’Toole, Consultant Urologist, RHC, Glasgow
- Martina Rodie, Consultant Neonatologist RHC, Glasgow
- Guftar Shaikh, Consultant Endocrinologist, RHC, Glasgow
Original version authors
- Suet Ching Chen, Paediatric Endocrinology Registrar, RHC, Glasgow
- Angela Lucas-Herald, Clinical Lecturer, RHC, Glasgow
- Martina Rodie, Clinical Lecturer, RHC, Glasgow
- Faisal Ahmed, Consultant Endocrinologist, RHC, Glasgow
- Alan Jackson, Consultant Neonatologist, PRM, Glasgow
- Andrew Powls, Consultant Neonatologist, PRM, Glasgow
Original version other specialists consulted
- Helen McDevitt, Consultant Neonatologist, RHC, Glasgow
- Ruth McGowan, Clinical Geneticist, South Glasgow Hospitals
- Jane McNeilly, Consultant Biochemist, RHC, Glasgow
- Guftar Shaikh, Consultant Endocrinologist, RHC, Glasgow
- Avril Mason, Consultant Endocrinologist, RHC, Glasgow
- Jarod Wong, Consultant Endocrinologist, RHC, Glasgow
- Ruth Hind, Clinical Psychologist, RHC, Glasgow
- Stuart O’Toole, Consultant Urologist, RHC, Glasgow
- Nicola Brindley, Consultant Surgeon, RHC, Glasgow
- Ruth Allen, Consultant Radiologist, RHC, Glasgow
Ahmed SF, Achermann J, Alderson J, Crouch NS, Elford S, Hughes IA, Krone N, McGowan R, Mushtaq T, O'Toole S, Perry L,
Rodie ME, Skae M, Turner HE. Society for Endocrinology UK Guidance on the initial evaluation of a suspected difference or
disorder of sex development (Revised 2021). Clin Endocrinol (Oxf). 2021 Dec;95(6):818-840.
Audí, L., Ahmed, S.F., Krone, N., Cools, M., McElreavey, K., Holterhus, P.M., Greenfield, A., Bashamboo, A., Hiort, O., Wudy, S.A. and McGowan, R., 2018. Genetics in endocrinology: approaches to molecular genetic diagnosis in the management of differences/disorders of sex development (DSD): position paper of EU COST Action BM 1303 ‘DSDnet’. European Journal of Endocrinology, 179(4), pp.R197-R206.
Lucas-Herald, A.K., Scougall, K. and Ahmed, S.F.. "Delivery of multidisciplinary care in the field of differences and disorders of sex development (DSD)." Expert Review of Endocrinology & Metabolism 17, no.3 (2022): 225-234.
Rodie, M.E., Ali, S.R., Jayasena, A., Alenazi, N.R., McMillan, M., Cox, K., Cassim, S.M., Henderson, S., McGowan, R. and Ahmed, S.F., 2022. A nationwide study of the prevalence and initial management of atypical genitalia in the newborn in Scotland. Sexual Development, 16(1), pp.11-18.
Last reviewed: 03 April 2024
Next review: 03 April 2027
Author(s): Angela Lucas-Herald, Clinical Lecturer, RHC, Glasgow; Faisal Ahmed, Consultant Endocrinologist, RHC, Glasgow; Emily Fraser, Psychologist, RHC, Glasgow; Ruth McGowan, Consultant Geneticist, QEUH, Glasgow; Jane McNeilly, Consultant Biochemist, QEUH, Glasgow; Stuart O’Toole, Consultant Urologist, RHC, Glasgow; Martina Rodie, Consultant Neonatologist RHC, Glasgow; Guftar Shaikh, Consultant Endocrinologist, RHC, Glasgow
Approved By: West of Scotland Neonatology Managed Clinical Network

