Extravasation injuries: prevention and management (neonatal guideline)
exp date isn't null, but text field is
Objectives
This guideline is applicable to all levels of nursing, midwifery and medical staff caring for neonates and infants in the West of Scotland. The purpose of this document is to guide staff in the prevention, early detection and management of extravasation injuries in the neonatal/ infant population. Staff must follow relevant guidelines: for aseptic techniques for procedures; for the insertion of Intravenous Cannulae (IVC); and the administration of Intravenous fluids and medicines. Staff caring for babies with peripheral venous cannulae should also utilize Peripheral Venous Cannulae (PVC) care bundles. Staff giving medications via peripheral venous cannulae should also follow the advice contained within the pharmacy drug monographs
Infiltration refers to the inadvertent administration of fluids or medications leak into the surrounding tissue instead of the intended vascular pathway.
Extravasation refers to the leakage of fluids or medications from a blood vessel leak into the surrounding tissues. This may occur secondary to occlusion of the vessel in which the cannulae is placed due to thrombophlebitis
Extravasation injury occurs when extravasated fluid causes injury to the surrounding tissues. This may occur more frequently when the fluid has an extreme pH (very acidic / alkaline), is hypertonic (high osmolality), is irritant, or is vasoactive. It may also occur if the extravasated fluid is confined within a restricted tissue compartment causing pressure on the blood vessels or nerves in the adjacent tissues (compartment syndrome)
Many babies, who are admitted to the Neonatal Unit and in particular to Neonatal Intensive Care, will require the insertion of an IVC. An IVC will usually be inserted for one of two reasons:
- For Intravenous (IV) medicine administration
- To deliver IV fluids (including blood and blood products)
Although a necessary therapeutic measure, the presence of an IVC is associated with several recognised complications, the most common being:
- Infection
- Infiltration / extravasation of infusate
Neonates are at high risk of extravasation injuries due to; reduced venous muscle tone, reduced vessel diameter and flexibility, easily distended subcutaneous tissue. They also have decreased peripheral circulation which increases the risk of medication / fluid pooling resulting in tissue damage.
Due to the increased flexibility of the subcutaneous tissue, damage can occur before extravasation is identified.
If these injuries are not detected and managed promptly and appropriately this can result in pain, infection, loss of function, nerve damage and permanent disfigurement/scarring. In many cases these injuries can be prevented or minimised by the careful observation and vigilance of the attending healthcare practitioner.
The pharmacological properties of a drug influence its ability to induce tissue damage. The following section identifies the medications most likely to result in extravasation injury
Vasoactive medicines
When vasoconstrictive medicines are administered peripherally, they may produce local vasoconstriction, leading to blanching of the skin around the infusion site. This effect may be extensive leading to severe tissue hypoxia and ischaemia of the surrounding tissues.
Vasodilators exacerbate extravasation injury by increasing local blood flow and enlarging the area of injury.
The following drugs have a high risk of causing tissue damage due to vasoconstriction or vasodilatation and should be given via the central route wherever possible. (exceptions would be in life- threatening emergency where no central venous access is available – In this case the line site should be checked very frequently e.g. at least every 15 minutes).
|
Medicine |
Examples of adverse effects |
Administration advice |
|
Adrenaline (epinephrine) |
Blanching of IV site – vasoconstriction |
Give by CVC |
|
Dinoprostone |
Vasodilatation |
Give by CVC |
|
Dobutamine |
Vasoconstriction or Vasodilatation |
Give by CVC |
|
Dopamine |
Blanching of IV site – vasoconstriction especially with large doses (May also cause Vasodilatation). |
Give by CVC |
|
Epoprostenol |
Vasodilatation |
Give by CVC |
|
Noradrenaline ( norepinephrine) |
Blanching of IV site - vasoconstriction |
Give by CVC |
|
Vasopressin |
Blanching of IV site - vasoconstriction |
Give by CVC |
Presence of excipients
Some drugs are formulated with substances such as polyethylene glycol and ethanol (alcohol) to improve their solubility, e.g. nimodipine. Such injectable medicines are known to be more irritating than those formulated in aqueous solutions.
|
Medicine |
Excipient content known to be irritant to veins |
|
Alprostadil |
Ethanol |
|
Clonazepam |
benzyl alcohol, ethanol, propylene glycol |
|
Co-trimoxazole |
ethanol, sodium metabisulphite |
|
Diazepam injection (soln) |
ethanol, propylene glycol |
|
Digoxin |
ethanol, propylene glycol |
|
Enoximone |
ethanol, propylene glycol |
|
Lorazepam |
propylene glycol |
|
Nimodipine |
alcohol, polyethylene glycol |
|
Omeprazole |
propylene glycol |
|
Phenobarbital |
disodium edetate, propylene glycol |
|
Phenoxybenzamine |
ethanol, polyethylene glycol |
Irritant medicines
The chemical properties of the drug may influence its propensity to cause tissue damage
Irritation due to extreme pH
Drug solutions with a pH <5.5 or pH >8.5 may cause tissue damage if they infiltrate subcutaneous tissue as they disturb the normal cellular environment. Blood and tissue fluid have a pH of 7.4 and deviation from this pH will cause damage to cellular structures, particularly by disturbing the function of proteins. The table below shows examples of medicines that have particularly high or low pH values. The reader should note pH values may vary slightly between different preparations of medicines, according to the manufacturer’s formulation.
The medicines (or strengths of medicines) in the following table marked * should be given centrally wherever possible
|
Intravenous medicine |
pH |
Intravenous medicine |
pH |
|
Aciclovir (above 5mg/ml *) |
11.3 |
Morphine |
3-6 |
|
Adrenaline * |
2.8-3.6 |
Naloxone |
3-4.5 |
|
Amikacin |
3.5-5.5 |
Noradrenaline * (norepinephrine ) acid tartrate |
3-4.5 |
|
Amiodarone * |
3-5 |
Octreotide |
3.9-4.5 |
|
Atropine |
2.8-4.5 |
Omeprazole |
9-10 |
|
Caffeine citrate |
4.7 |
Ondansetron |
3.3-4.0 |
|
Clonazepam |
3.5-4.5 |
Parenteral nutrition (see local policy*) |
varies |
|
Dantrolene |
9.5 |
Phenobarbital (phenobarbitone) |
9-10.5 |
|
Dobutamine * |
2.5-5.5 |
Phenytoin sodium |
12 |
|
Dopamine * |
2.5-5.5 |
Potassium canrenoate |
10.7-11.2 |
|
Fentanyl |
4.0-7.5 |
Potassium salts (above 40mmol/L*) |
Varies |
|
Filgrastim |
4 |
Propranolol |
3 |
|
Flumazenil |
3.8-4.5 |
Protamine sulphate |
2.5-3.5 |
|
Furosemide |
8-9.5 |
Pyridoxine |
2.0-3.8 |
|
Ganciclovir * |
10-11 |
Quinine dihydrochloride |
1.5-3.0 |
|
Gentamicin |
3-5 |
Rifampicin (6mg/ml* ) |
8.3 |
|
Glucagon |
2.5-3.5 |
Rocuronium |
3.8-4.2 |
|
Glucose (pH dependent on Concentration of solution) |
3.5-6.5 |
Salbutamol |
3.5 |
|
Hydralazine |
3.5-4.2 |
Sodium nitroprusside |
3.5-6.0 |
|
Isoprenaline |
3.5-4.5 |
Suxamethonium |
3.0-4.5 |
|
Ketamine |
3.5-5.5 |
Tetracosactide |
3.8-4.5 |
|
Labetalol |
3.5-4.2 |
Thiopental * |
10.5 |
|
Lidocaine |
3.5-6.0 |
Tobramycin |
3.5-6.0 |
|
Liothyronine |
9.8-11.2 |
Vancomycin ( 20mg/ml*) |
2.8-4.5 |
|
Midazolam |
3 |
|
|
|
Milrinone |
3.2-4.0 |
|
|
Tonicity
All solutions exert an osmotic pressure, dependent on the amount of substance dissolved in the solution. The tonicity of a solution is measured relative to water, which has an osmolarity of 0 mOsmol/L. Solutions with an osmolarity more or less than that of plasma (~290 mOsmol/L) may cause tissue damage. The presence of these solutions can lead to an osmotic imbalance across the cell membrane, leading to the movement of water into or out of the cell, a breakdown of cellular transport mechanisms and cell death. Most injectables are formulated to have the same osmotic pressure as plasma so that the solution to be injected into the patient is unlikely to cause vein irritation. The table below lists a selection of medicines that have high osmolarity and may potentially cause a problem if extravasated. Extra care should be taken when administering these medicines .
Few medicines have an osmotic pressure less than plasma; however, if a medicine is made up with greater than the recommended volume of water for injections, the medicine is likely to be hypotonic and may cause tissue irritation. In practice, this tends to be much less of an issue than injection of hypertonic solutions.
Osmolarity or osmolality? What is the difference?
Tonicity is stated using two different conventions: osmolarity is the theoretical tonicity and is derived through calculation. Osmolality is the measured tonicity and is derived through laboratory testing, such as freezing point depression. Both values are usually similar, with some notable exceptions such as calcium gluconate. This has an osmolarity of 670 mOsmol/L and an osmolality of 276 mOsmol/kg. Some medicines may be more irritating to tissues than expected, based on based on their osmolarity. Where possible the osmolality of a solution is stated in the relevant pharmacy information, as this is a better indicator of whether a medicine will cause tissue damage. If a medicine has an osmolality of greater than 500 mOsmol/kg (or an osmolarity of greater than 500 mOsmol/L ) it is more likely to cause problems if it infiltrates a tissue. This risk increases with increasing osmolalityabove this value. Solutions with an osmolarity of > 1000 mOsmol/L should never be infused peripherally.
Medicines with a high tonicity may be diluted to a larger volume of infusion fluid in order to reduce the tonicity and thus reduce the irritancy of the medicine.
The following table lists fluids with an osmolality of >500 mOsmol/L for reference
|
Intravenous medicine |
Osmolarity |
Intravenous medicine |
Osmolarity |
|
Glucose 10% |
535 |
Potassium chloride |
4000 |
|
Glucose 12.5% |
669 |
Sodium benzoate above 50mg/ml |
varies |
|
Glucose 15% |
802 |
Sodium bicarbonate 4.2% |
1004 |
|
Glucose 20% |
1110 |
Sodium bicarbonate 8.4% |
2008 |
|
Calcium gluconate 10% |
670 |
Sodium chloride 1.8% |
616 |
|
Calcium chloride |
1500 |
Sodium chloride 2.7% |
924 |
|
Parenteral nutrition |
(variable with bag contents) |
Sodium phenylbutyrate above 50mg/ml |
varies |
|
Magnesium sulphate 50% |
4060 |
X ray contrast media |
varies |
|
Mannitol 10% |
550 |
|
|
|
Mannitol 20% |
1100 |
|
|
Registered Health Care Practitioners are responsible for:
- Informing parents/carers of the injury in the event of tissue damage.
- Liaising with parents/carers and the interdisciplinary team to promote compliance with guidance, ensuring that Interdisciplinary Patient Focused Care Plans are in place and interventions are recorded and dated in line with the Board’s Record Keeping Policy.
- Maintaining and updating their knowledge, skills and competence in line with their roles and responsibilities to care for infants who sustain an injury.
- Seeking the advice of the Medical staff and or Tissue Viability Service where appropriate, whilst maintaining ongoing responsibility for the infant’s episode of care.
- Document injury in nursing notes, commence a wound assessment chart and appropriate interventional care plan
- Insert information into the unit Extravasation Safety Cross as per guidance for all injuries Stage C and above.
- Complete a Datix for all injuries Stage C and above.
- Refer all injuries that result in soft tissue damage to Medical staff and Tissue Viability Nurse.
- Obtain clinical photographs for all injuries Stage C and above ensuring appropriate consent is obtained
Setting Pump Pressure Alarms
Where clinically acceptable it is best practice to set infusion in-line pressure alarm settings using the “Auto Set Pressure” feature on the infusion pump. This may be used once a stable pressure has been achieved (over approximately first 10 minutes of infusion) to set pressure 30mmHg above the average in-line pressure allowing for a quicker ‘time to alarm’.
This will alert the healthcare practitioner supporting early clinical assessment and intervention which may assist in reducing the risks of complications. All sudden increases in pressure or increasing pressure trends must be investigated and the infusion line and IVC site examined carefully.
i. Causes of extravasation and Prevention
|
Cause of injury |
Why did this happen |
How to prevent |
|
Dislodged device |
Poorly secured/during securing of the cannula. Accidental pulling on IV administration set. Patient movement. |
Secure device well with a sterile transparent dressing. Ensure lines are not trapped during patient handling, use of incubator. |
|
Venous irritation |
Irritation causes vasoconstriction, leads to back pressure, expanding insertion site and leakage of fluid. |
Observe for sudden change in infusion pressure. Administer bolus injection and or flush slowly. |
|
Through and through puncture of vein |
Cannula insertion technique - cannula penetrates through the vein but practitioner pulls it back and inserts into vein (hole remains) |
If practitioner feels they have gone though the vein they must chose a different insertion site. Confirm placement by flushing with a small amount of 0.9% Sodium Chloride. |
|
Cannula insertion site |
Choosing a site below previous insertion site that has not healed can cause leakage of fluid. Areas of flexion can lead to dislodged IVCs. |
Avoid insertion below recent cannula insertion sites using the same vein. Ensure joint is kept in extended position using a splint (take care not to obscure for easy inspection). |
|
Formation of thrombus at the line tip leading to back flow/ruptured venous wall |
A small clot forming at the tip of the cannula |
Observe for a gradual increase in infusion device pressure |
|
Insertion of IVC unintentionally into an artery |
Poor insertion technique. This will be apparent if there is; blanching of the limb, pulsation of blood into cannula/ extension set. |
|
ii. Observation
|
Do |
Why? |
|
Use an sterile adhesive transparent film dressing to secure the IVC |
To secure the device, ensure easy inspection of the site and reduce risk of infection |
|
Observe insertion site and surrounding skin hourly and document findings in clinical notes |
To ensure early detection of changes/possible injury and provide evidence of care |
|
Observe digits distal to insertion site hourly (if limb vein in use) |
Swelling may occur distally to the insertion site and can result in compartment syndrome. A thrombus may have formed resulting in limb ischaemia. |
|
Record infusion device baseline alarm limits and record pressures hourly |
To allow for early detection in rise in pressure and provide evidence of care. A gradual increase in pressure may indicate extravasation / thrombus formation. A sudden rise in pressure may indicate IV administration line occlusion. |
|
Act on any increase in infusion device pressure |
This may be due to extravasation or thrombus formation |
|
If a bolus injection is being administered: check cannula patency with 0.9% Sodium Chloride prior to medication administration |
If vein is not intact this will lead to infiltration only as 0.9% Sodium Chloride is non-vesicant |
|
If a bolus injection is being administered: observe site before, during and after administration for blanching, erythema or leakage of fluid |
To observe for signs if injury during administration |
|
Administer vesicant drugs before non-vesicant |
Staff assessment of vein patency is considered more accurate at the start of administration |
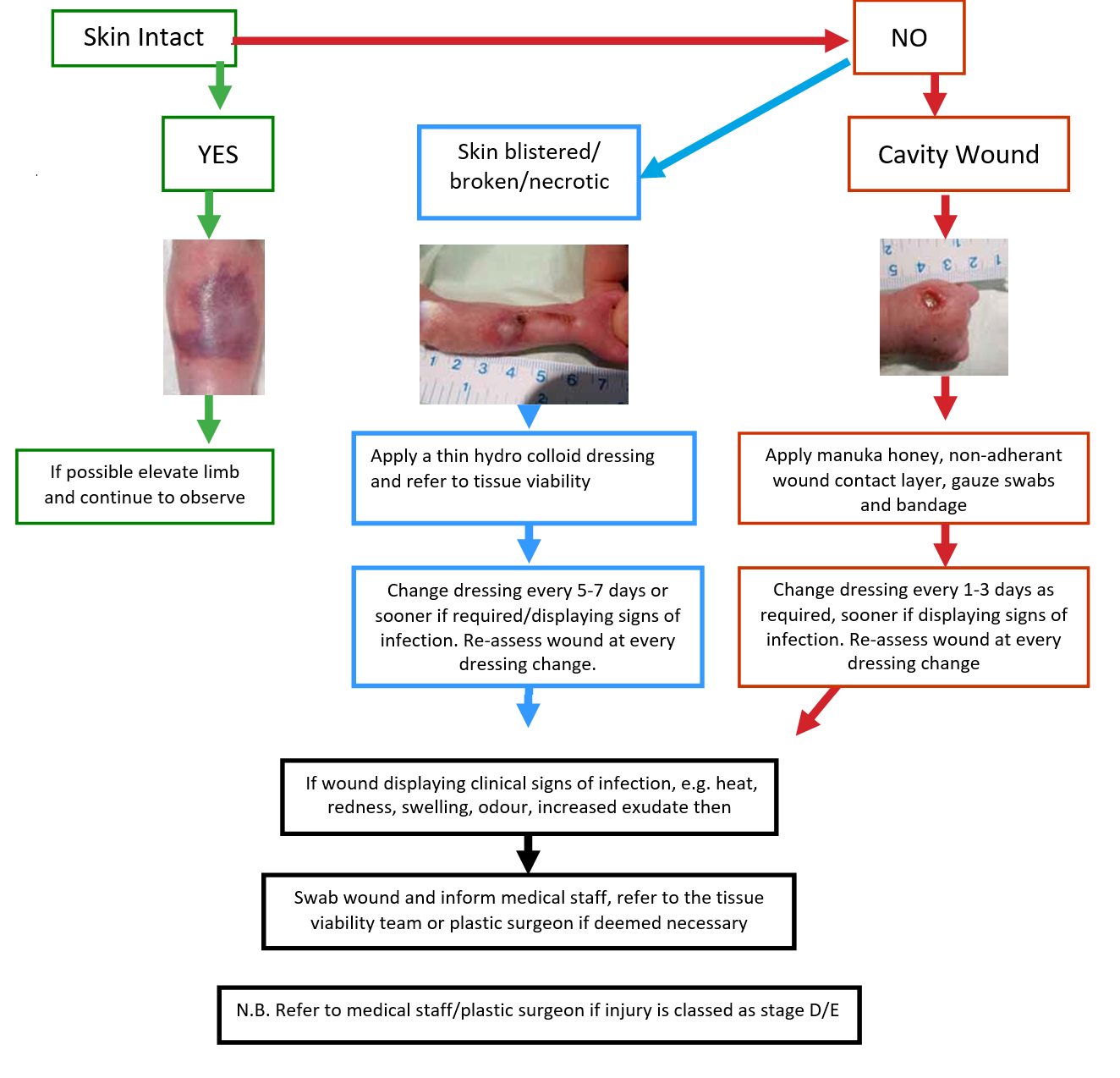
iii. Staging and Management of Infiltration/Extravasation Injuries (Adapted from Flemmer & Chan, 1993)
N.B. Not all symptoms will be present at each stage
|
STAGE |
CHARACTERISTICS |
RECOMMENDED ACTION |
|
A |
|
|
|
B |
|
|
|
C |
|
|
|
D |
|
|
|
E |
|
|
Irrigation
A Cochrane review (Gopalakrishnan et al, 2012) concluded that there was insufficient evidence to examine the effects of irrigation with or without hyaluronidase in the management of extravasation injuries in neonates. Indeed, if inexpertly performed, irrigation may worsen the injury. Irrigation would never be considered appropriate for low grade (A-C) extravasations. If irrigation is deemed to be necessary after clinical assessment of the baby it must be carried out by a suitably experienced Neonatologist or Plastic Surgeon as this can cause the onset of compartment syndrome amongst other complications.
Provide wound products for one weeks wound management.
- Dougherty, L. (2010) “Extravasation: Prevention, Recognition and Management.” Nursing Standard, vol. 24, no. 52, pp. 48-55.
- Flemmer, L. & Chan, J. (1993) A Paediatric Protocol for Management of Extravasation Injuries. Paediatric Nursing. 18, pp. 44-47.
- Glass, & Glacoia, G.P. (1997) Intravenous drug therapy in premature infants and children. Journal of obstetrics, gynaecology and neonatal
- Vol. 16, pp 310-318.
- Gopalakrishnan, N., Goel, N. and Banerjee, S. (2012) “Saline irrigation for the management of skin extravasation injury in neonates (Review).” Cochrane Database of Systematic reviews, Issue 2. Art. No.: CD008404. DOI: 10.1002/14651858. CD008404.pub2.
- Jonckers,, Berger, I., Kuijten, T, Meijer, E. and Andriessen, P. (2014) “The effect of in-line infusion filtering on in-line pressure monitoring in an experimental infusion system for newborns.” Neonatal Network, vol. 33, no. 3, pp. 133-137.
- McCullen, K.L. & Pieper, B. (2006) A retrospective chart review of risk factors for extravasation among neonates receiving peripheral intravascular Journal of Wound Ostomy and Continence Nursing. Vol. 33(2), pp. 133-139.
- Nursing & Midwifery Council (2002) Guidelines for records and record keeping.
- Rosenthal, K. (2007) Reducing the risks of infiltration and extravasation. Nursing (Med/Surg Insider). 37, pp 4,6-8.
- Wynsma, L.A. (1998) Negative outcomes of intravascular therapy in infants and children. AACN Critical Care: Advanced Practice in Acute & Critical Vol. 9(1), pp. 49-63.
- Hadaway, Infiltration and Extravasation. American Journal of Nursing August 2007.
- M G Gnanalingham, V Irving, N J Consensus on neonatal infusion pumps and pressure monitoring. Archives of Disease in Childhood Fetal and Neonatal edition 2005
- Reynolds, B. (2007) “Neonatal extravasation injury: Case report.” Infant, vol. 3, no. 6, pp. 230-232.
- Dawn Camp Developing Extravasation Protocols and Monitoring Outcomes. Journal of Intravenous Nursing July/August 1998 (Weinstein 2007, Hyde and Dougherty, 2008).
Last reviewed: 05 November 2019
Next review: 01 November 2022
Author(s): Angela Rodgers – Tissue Viability Nurse GG&C; Gillian Frew – Neonatal Nurse PRM
Co-Author(s): Other Professionals Consulted: June Grant – Neonatal Pharmacist PRM
Approved By: West of Scotland Neonatology Managed Clinical Network