Audiological investigation in cystic fibrosis patients
exp date isn't null, but text field is
Objectives
This guideline describes referral, monitoring and follow-up pathways for audiological screening of children with cystic fibrosis (CF) at RHC Glasgow who may have received IV or Nebulised aminoglycosides or oral Macrolides as part of their treatment. The aim of the Guideline is to allow for early detection of hearing loss in these children with referral to Audiological Specialist services where required.
Scope
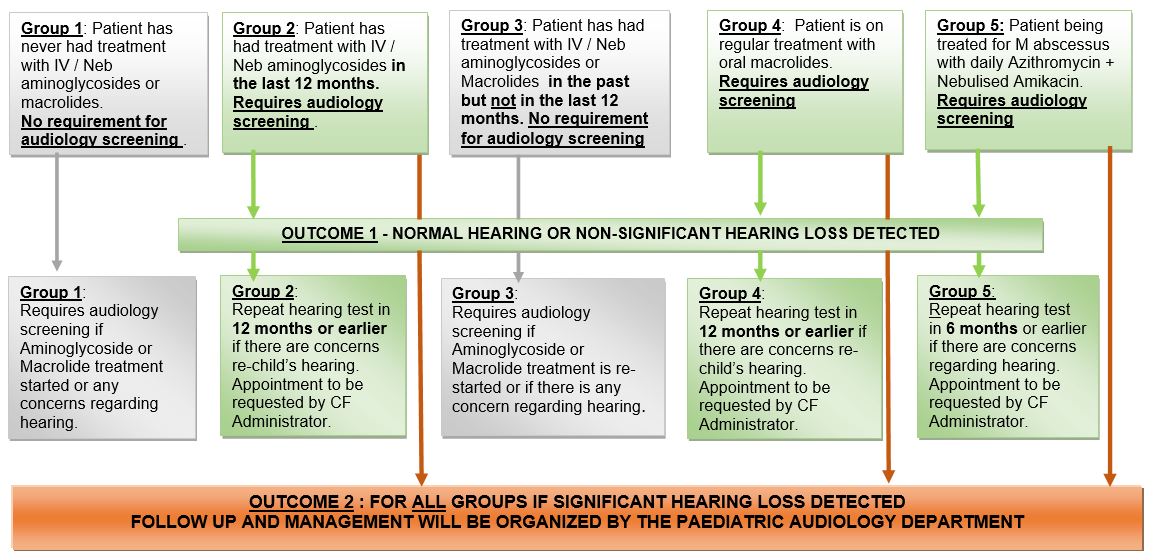
Children with CF attending RHC Glasgow who may receive IV Aminoglycosides or oral Macrolides.
Audiological screening is recommended for patients with Cystic Fibrosis (CF) in order to monitor potential drug induced ototoxicity following Aminoglycoside and Macrolide administration 1,2
AMINOGLYCOSIDE USE IN CF
Children with CF may require several courses of IV Aminoglycosides per year and some patients require daily nebulised Aminoglycosides longer-term.
Aminoglycoside antibiotics, however administered, have been shown to be associated with ototoxicity 1. Classical findings are of high frequency hearing loss =/- vestibular symptoms.
MACROLIDES USE IN CF
Children with CF are often prescribed oral Azithromycin for treatment of infections and as an anti-inflammatory agent long-term. There is evidence 2 that long-term use of Macrolide antibiotics may be associated with sensorineural hearing loss. We have arranged that CF patients receiving regular Macrolide therapy undergo audiological screening outlined below.
M ABSCESSUS TREATMENT
Children with CF who are undergoing treatment for M abscessus lung disease will be receiving daily Nebulised Aminoglycosides (usually Amikacin) and an oral Macrolide. They may also be receiving intermittent IV Aminoglycosides. These patients require to have audiological assessment at least 6 monthly.
Although assays for IV Aminoglycoside levels are monitored closely, published literature has highlighted evidence that there may be a small percentage of patients who may exhibit enhanced susceptibility to Aminoglycoside-induced ototoxicity, even at normal serum assay levels.
This susceptibility may be genetically determined and may predispose to hearing impairment many years after initial exposure to Aminoglycoside.
[Fischel-Ghodsian et al UCLA 2004]
- A1555G / delT961Cn in the mitochondrial 12s ribosomal RNA gene
- Multiple studies reporting presence of A1555G in patients with sensorineural hearing loss associated with Aminoglycoside antibiotics.
- 17% A1555G +ve in study of 41 adults exhibiting hearing impairment post-Aminoglycosides
[Fischel-Ghodsian et al UCLA 2004]
- 17% A1555G +ve in study of 41 adults exhibiting hearing impairment post-Aminoglycosides
- A1555G mutation also reported in hearing-impaired patients with NO history of Aminoglycoside exposure
[Tang et al 2002] - Overall population prevalence of A1555G and delT196Cn
- AI555G 09%
- DelT961Cn 6%
[Tang et al ; Texas 2002]
Ongoing research may be informative for the co-utilisation of protective measures in genetically susceptible individuals who, for clinical reasons, would be disadvantaged by the cessation of all Aminoglycoside therapy. 3
Genetic screening for AI555G mutation will be carried out as part of first Annual Review investigations for patients with Cystic Fibrosis attending RHC.
- Rizzi, Mark Douglas; Hirose, Keiko. Aminoglycoside ototoxicity. Current Opinion in Otolaryngology & Head & Neck Surgery. 15(5):352-357, 2007.
- Westerberg, Mick P. Sensorineural hearing loss as a probable serious adverse drug reaction associated with low-dose oral azithromycin. Journal of Otolaryngology. 36(5):257-63, 2007.
- Tien Nguyen, Anita Jeyakumar. Genetic susceptibility to aminoglycoside ototoxicity. International Journal of Pediatric Otorhinolaryngology. Vol 120, May 2019, pp15-19.
Last reviewed: 14 December 2020
Next review: 31 May 2025
Author(s): Dr Jane Wilkinson; Dr Juan Mora; Mr Jim Harrigan; Kirstin Marchbanks
Version: 1
Approved By: Paediatric Clinical Effectiveness & Risk Committee
Document Id: 1031