Omalizumab: difficult asthma advice note
exp date isn't null, but text field is
Objectives
Advice Note for Asthma Clinics in the West of Scotland
(For use in a fully assessed and monitored patient as part of a difficult asthma service)
Over 50% of patients with severe asthma have allergic (IgE-mediated) asthma 1. Omalizumab, a humanised monoclonal antibody manufactured by recombinant DNA technology, is the first therapeutic agent to bind free IgE and inhibit mast cell degranulation. It reduces free IgE by almost 95% by binding to the CЄ3 region on free IgE and blocks it binding to its specific high-affinity receptor 2. Omalizumab reduces serum IgE levels and receptor expression on key cells in the inflammatory cascade, limits the release of inflammatory mediators from mast cells and reduces the infiltration of inflammatory cells, especially eosinophils, into the airway 2.
Omalizumab is a therapeutic option for patients with allergic asthma where the serum IgE levels are 30-1500 IU/ml, for ages six and above. The injection is administered subcutaneously every 2 or 4 weeks, calculated by using the combination of total IgE level and body weight.
Efficacy
Efficacy and safety have been studied in many randomised, placebo-controlled trials and this drug is now recommended by the British Guidelines on Asthma Management 3 as well as the international GINA Guidelines for patients at step 5 of treatment 4 and by the US Expert Panel on asthma at steps 5 and 6 of treatment 5.
A Cochrane review in 2006 showed that the addition of omalizumab to usual therapy is effective in moderate-severe allergic asthma with a significant reduction in inhaled corticosteroids and rescue medication dose, but no reduction in exacerbations in patients with severe asthma requiring oral corticosteroids 6. A recent systematic review by Rodrigo and colleagues studied eight large clinical trials (3429 participants) 7. Patients on omalizumab had a decreased risk of asthma exacerbations at the end of stable (RR 0.57, 95% CI, 0.48-0.66; p=0.0001) and adjustable steroid phases (RR 0.55, 95%CI, 0.47-0.64;p=0.0001). At the end of steroid reduction phase [inhaled and oral corticosteroids], subjects treated with omalizumab were more likely to withdraw from corticosteroids completely compared to placebo (RR 1.8, 95%CI, 1.42-2.28; p=0.00001). These were the major changes with treatment, with secondary outcomes of changes in lung function (FEV1 and PEF) being minimal.
Safety
The safety profile of omalizumab has been analysed from clinical studies of over 7500 patients and by post-marketing safety data on 57,300 patients and has been shown to be favourable and the drug well tolerated 8. Incidence of anaphylaxis was 0.14% in omalizumab treated patients and 0.07% in control patients in clinical trials and 0.2% in the post-marketing data 8. In the systematic review of safety by Rodrigo et al, serious side effects were similar in omalizumab (3.8%) and placebo (5.3%) 7. Injection site reactions were commoner in omalizumab treated patients (19.9% compared to 13.2%).
Anaphylactic reaction risk was 0.33% in omalizumab vs 0.24% in placebo (RR=1.08, 95%CI 0.13-8.74; p=0.94) 7.
The FDA and MHRA have recently raised concerns about cardiovascular and cerebrovascular safety of omalizumab based on preliminary data from a five year epidemiological study designed to evaluate the clinical effectiveness and long-term safety in patients with moderate-to-severe asthma (EXELS)9. Final safety results from the EXCELS study are awaited.
Due to the high cost of this treatment, the Scottish Medicines Consortium [SMC] and NICE have added patient selection criteria to maximise benefit in a carefully selected population of patients.
SMC and NICE Advice
Omalizumab (Xolair®) is accepted for use within Scotland as add-on therapy to improve asthma control in patients (6 years of age and above) with severe persistent allergic asthma. It is restricted to initiation and monitoring by hospital physicians experienced in the diagnosis and treatment of severe persistent asthma. The response to omalizumab treatment should be assessed in all patients at 16 weeks and treatment should be discontinued in patients who have not shown a marked improvement in overall asthma control.
Eligibility criteria
- Assessment by a hospital physician experienced in the diagnosis and treatment with severe persistent allergic asthma
- Positive skin test/serology to perennial aero-allergen and total serum IgE between 30 and 1500 IU/ml
- Adult and adolescent patient (6 years of age and above)reduced lung function (forced expiratory volume at 1 second [FEV1] less than 80%
- frequent daytime symptoms or night-time awakenings
- multiple documented severe exacerbations despite daily high-dose inhaled corticosteroids plus a long-acting inhaled beta2 agonist
AND
- continuous or frequent treatment with oral corticosteroids (defined as 4 or more courses in the previous year)
Optimised standard therapy is defined as a full trial of and, if tolerated, documented compliance with inhaled high-dose corticosteroids, long-acting beta2 agonists, leukotriene receptor antagonists, theophyllines, oral corticosteroids and smoking cessation if clinically appropriate.
Dose: Calculate dose as per Chart in Appendix 1. Patient cannot be treated if total IgE and weight combination does not fall within the dosing chart options.
Ensure patient meets the inclusion criteria for treatment.
After a detailed discussion of the treatment and potential side effects, start the run-in period of 4 weeks.
Run-in period:
- Stable dose of oral steroids
- No exacerbations [consider repeating run-in phase if exacerbation occurs]
- ACT, mini AQLQ forms
- Spirometry
- Exhaled nitric oxide
- Record numbers of exacerbations and steroid use in the past year
- Give peak flow meter for recording morning PEF. Minimum readings are 3 in the week before treatment is initiated.
Baseline/Start of Omalizumab treatment
- Check criteria met
- Confirm dose by checking IgE and weight
- Spirometry
- Exhaled NO
- ACT, mini AQLQ forms
- Explain risk of anaphylaxis
- Give first injection
- Observe for at least 2 hours for allergic reactions.
- Send home with a letter regarding start of treatment, plan of action if anaphylaxis symptoms occur, next appointment [2 or 4 weeks later for injection;2 months for clinic].
- Continue peak flow readings [at least for the week prior to clinic assessment]
If there is a history of previous anaphylaxis, prescribe and explain use of adrenaline autoinjectors such as epipens. Since anaphylaxis is a rare side effect, the consensus agreement of the West of Scotland Asthma specialists, in consultation with the local Anaphylaxis service, is that routine provision of adrenaline to every patient prescribed omalizumab is not essential.
Method of administration of injection:
For subcutaneous administration only.
Do not administer by the intravenous or intramuscular route.
The injections are administered subcutaneously in the deltoid region of the arm. Alternatively, the injections can be administered in the thigh if there is any reason precluding administration in the deltoid region. Details in Appendix 4.
Waiting time after injection:For patients with no previous history of anaphylaxis, the minimum waiting time after the first injection should be 2 hours, after injections 2-4 it should be 1 hour and if there have been no side effects, the waiting time thereafter could be 30 minutes. Individualize If history of anaphylaxis in the past to other agents.
At 2 months: [optional]
- Spirometry
- Exhaled NO
- ACT, mini AQLQ forms
- Continue peak flow readings [at least for the week prior to clinic assessment]
At 4 months
- Spirometry
- Exhaled NO
- ACT, mini AQLQ forms
- GETE Score by patient and physician
- Decision made whether to continue or omit omalizumab.
If continuing, attempt to taper oral prednisolone slowly.
Next clinic appointment after 4 months.
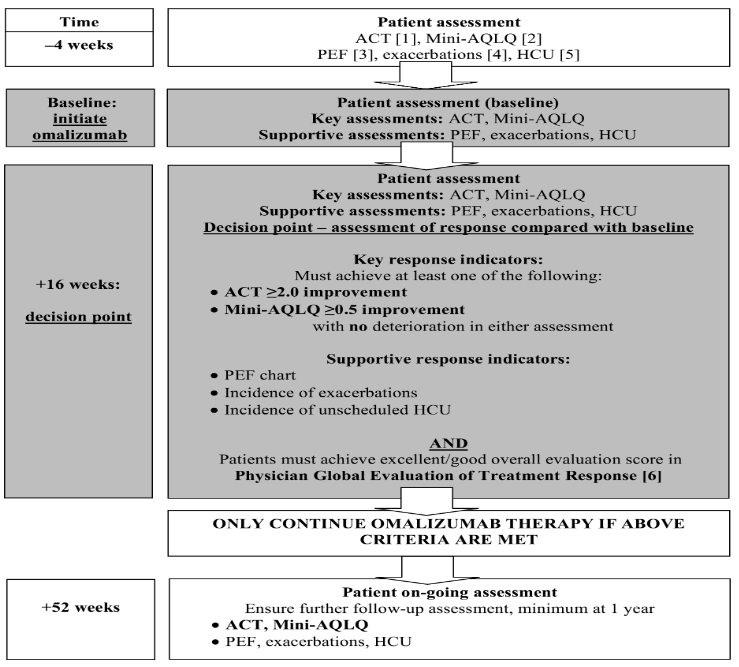
Protocol for the Assessment of Response to Treatment with Omalizumab
[1] ACT: Asthma Control Test, 5-item, self-administered survey, covers previous 4 weeks.
[2] Mini-AQLQ: Mini Asthma Quality of Life Questionnaire, 15 items, covers previous 2 weeks.
[3] PEF: Peak Expiratory Flow, performed within 15 minutes of waking, on Mon, Wed, Fri.
[4] Exacerbations: asthma worsening episodes requiring use of additional oral corticosteroids. Patients should be exacerbation free for 4 weeks before baseline assessment.
[5] HCU: Unscheduled Healthcare Utilisation: hospitalization for asthma; A&E attendance; GP visit
[6] Physician Global Evaluation Of Treatment Effectiveness: an overall clinical evaluation of improvement in asthma control at 16 weeks compared to baseline, based on all available information: patient interview, review of patient notes & diary (if used), and key & supportive response indicators. 5-level evaluation graded
|
Excellent |
(complete control of asthma) |
|
Good |
(marked improvement of asthma) |
|
Moderate |
(discernible, but limited improvement in asthma) |
|
Poor |
(no appreciable change in asthma) |
|
Worsening |
(of asthma) |
Excellent/good evaluation indicates response to omalizumab treatment.
Disease register
- Register kept of all patients started on treatment and of those continuing treatment after 16 weeks.
|
DATE |
|
|
|
|
||||
|
ASSESSMENTS |
Visit 1 |
Visit 2 |
Visit 3 |
Visit 4 |
||||
|
|
- 4 weeks before Rx |
Baseline, 1 month later |
2 months after Rx |
4 months after Rx |
||||
|
Detailed discussion with patient |
x |
|
x |
|
|
|
|
|
|
Oral steroid dose stabilised |
x |
|
x |
|
x |
|
x |
|
|
FEV1
|
x |
|
x |
|
x |
|
x |
|
|
Exhaled NO |
x |
|
x |
|
x |
|
x |
|
|
Asthma Control Test Score
Change from baseline |
x |
|
x |
|
x |
|
X
x |
|
|
Mini-AQLQ Score
Change from baseline |
x |
|
x |
|
|
|
X
x |
|
|
Any side effects?
|
|
|
|
|
x |
|
x |
|
|
Courses of oral corticosteroids use or increase? |
x |
|
x |
|
x |
|
x |
|
|
Any asthma exacerbation? |
|
|
x |
|
x |
|
x |
|
|
GETE Score for efficacy by patient |
|
|
|
|
|
|
x |
|
|
GETE Score for efficacy by physician |
|
|
|
|
|
|
x |
|
If treatment to continue after 4 months, then assess at clinic every 4 months Attempt to reduce oral corticosteroids if possible.
Subsequent visit schedule at clinic
- 8 months after Rx
- 1 year after Rx – assess whether to continue based on similar criteria
- 1 year, 4 months
- 1 year, 8 months
- 2 years after Rx- assess whether to continue based on similar criteria
- 2 years, 4 months
- 2 years, 8 months
- 3 years after Rx- assess whether to continue based on similar criteria.
To consider if a trial of omission of Rx can be attempted.
ADMINISTRATION EVERY 2-4 WEEKS. Xolair doses (milligrams per dose) administered by subcutaneous injection
Body Weight (kg) |
|||||||||||
Baseline IgE (IU/ml) |
20-25 | >25-30 | >30-40 | >40-50 | >50-60 | >60-70 | >70-80 | >80-90 | >90-125 | >125-150 | |
| ≥30-100 | 75 | 75 | 75 | 150 | 150 | 150 | 150 | 150 | 300 | 300 | |
| >100-200 | 150 | 150 | 150 | 300 | 300 | 300 | 300 | 300 | 450 | 600 | |
| >200-300 | 150 | 150 | 225 | 300 | 300 | 450 | 450 | 450 | 600 | 375 | |
| >300-400 | 225 | 225 | 300 | 450 | 450 | 450 | 600 | 600 | 450 | 525 | |
| >400-500 | 225 | 300 | 450 | 450 | 600 | 600 | 375 | 375 | 525 | 600 | |
| >500-600 | 300 | 300 | 450 | 600 | 600 | 375 | 450 | 450 | 600 | ||
| >600-700 | 300 | 225 | 450 | 600 | 375 | 450 | 450 | 525 | |||
| >700-800 | 225 | 225 | 300 | 375 | 450 | 450 | 525 | 600 | |||
| >800-900 | 225 | 225 | 300 | 375 | 450 | 525 | 600 | ||||
| >900-1000 | 225 | 300 | 375 | 450 | 525 | 600 | |||||
| >1000-1100 | 225 | 300 | 375 | 450 | 600 | 4-weekly dosing schedule | |||||
| >1100-1200 | 300 | 300 | 450 | 525 | 600 | ||||||
| >1200-1300 | 300 | 375 | 450 | 525 | 2-weekly dosing schedule | ||||||
| >1300-1500 | 300 | 375 | 525 | 600 | |||||||
Note:
Treatment duration, monitoring and dose adjustments
Discontinuation of Xolair treatment generally results in a return to elevated free IgE levels and associated symptoms. Total IgE levels are elevated during treatment and remain elevated for up to one year after the discontinuation of treatment. Therefore, re-testing of IgE levels during Xolair treatment cannot be used as a guide for dose determination. Dose determination after treatment interruptions lasting less than one year should be based on serum IgE levels obtained at the initial dose determination. Total serum IgE levels may be re-tested for dose determination if treatment with Xolair has been interrupted for one year or more.
Doses should be adjusted for significant changes in body weight
Special populations
Elderly (65 years of age and older): There are limited data available on the use of Xolair in patients older than 65 years but there is no evidence that elderly patients require a different dose from younger adult patients.
Renal or hepatic impairment: There have been no studies on the effect of impaired renal or hepatic function on the pharmacokinetics of Xolair. Because omalizumab clearance at clinical doses is dominated by the reticular endothelial system (RES) it is unlikely to be altered by renal or hepatic impairment. While no particular dose adjustment is recommended for these patients, Xolair should be administered with caution.
Paediatric population: The safety and efficacy of Xolair in children below age 6 have not been established. No data are available.
[Always review latest online Summary of Product Characteristics]
|
Infections and infestations |
|
|
Uncommon |
Pharyngitis |
|
Rare |
Parasitic infection |
|
Blood and lymphatic system disorders |
|
|
Not known |
Idiopathic severe thrombocytopenia |
|
Immune system disorders |
|
|
Rare |
Anaphylactic reaction, other serious allergic conditions |
|
Not known |
Serum sickness, may include fever and lymphadenopathy |
|
Nervous system disorders |
|
|
Common |
Headache* |
|
Uncommon |
Syncope, paraesthesia, somnolence, dizziness |
|
Vascular disorders |
|
|
Uncommon |
Postural hypotension, flushing |
|
Respiratory, thoracic and mediastinal disorders |
|
|
Uncommon |
Allergic bronchospasm, coughing |
|
Rare |
Laryngoedema |
|
Not known |
Allergic granulomatous vasculitis (i.e. Churg-Strauss syndrome) |
|
Gastrointestinal disorders |
|
|
Common |
Abdominal pain upper** |
|
Uncommon |
Dyspeptic signs and symptoms, diarrhoea, nausea |
|
Skin and subcutaneous tissue disorders |
|
|
Uncommon |
Photosensitivity, urticaria, rash, pruritus |
|
Rare |
Angioedema |
|
Not known |
Alopecia |
|
Musculoskeletal and connective tissue disorders |
|
|
Not known |
Arthralgia, myalgia, joint swelling |
|
General disorders and administration site conditions |
|
|
Very common |
Pyrexia** |
|
Common |
Injection site reactions such as swelling, erythema, pain, pruritus |
|
Uncommon |
Influenza-like illness, swelling arms, weight increase, fatigue |
*: Very common in children 6 to <12 years of age
**: In children 6 to <12 years of age
Immune system disorders
- Allergic reactions type I : Type I local or systemic allergic reactions, including anaphylaxis and anaphylactic shock, may occur when taking omalizumab, also with onset after a long duration of treatment. Most of these reactions occurred within 2 hours after the first and subsequent injections of Xolair but some started beyond 2 hours and even beyond 24 hours after the injection. Therefore medicinal products for the treatment of anaphylactic reactions should always be available for immediate use following administration of Xolair. Patients should be informed that such reactions are possible and prompt medical attention should be sought if allergic reactions occur.
Anaphylactic reactions were rare in clinical trials.
- Serum sickness : Serum sickness and serum sickness-like reactions, which are delayed allergic type III reactions, have been seen in patients treated with humanised monoclonal antibodies including omalizumab. The suggested pathophysiologic mechanism includes immune-complex formation and deposition due to development of antibodies against omalizumab. The onset has typically been 15 days after administration of the first or subsequent injections, also after long duration of treatment. Symptoms suggestive of serum sickness include arthritis/arthralgias, rash (urticaria or other forms), fever and lymphadenopathy. Antihistamines and corticosteroids may be useful for preventing or treating this disorder, and patients should be advised to report any suspected symptoms.
- Churg-Strauss syndrome and hypereosinophilic syndrome : Patients with severe asthma may rarely present systemic hypereosinophilic syndrome or allergic eosinophilic granulomatous vasculitis (Churg-Strauss syndrome), both of which are usually treated with systemic corticosteroids.
In rare cases, patients on therapy with anti-asthma medicinal products, including omalizumab, may present or develop systemic eosinophilia and vasculitis. These events are commonly associated with the reduction of oral corticosteroid therapy.
In these patients, physicians should be alert to the development of marked eosinophilia, vasculitic rash, worsening pulmonary symptoms, paranasal sinus abnormalities, cardiac complications, and/or neuropathy.
Discontinuation of omalizumab should be considered in all severe cases with the above mentioned immune system disorders.
Arterial thromboembolic events (ATE): In controlled clinical trials and during interim analyses of an observational study, a numerical imbalance of ATE was observed. ATE included stroke, transient ischaemic attack, myocardial infarction, unstable angina, and cardiovascular death (including death from unknown cause). In the final analysis of the observational study, the rate of ATE per 1,000 patient years was 7.52 (115/15,286 patient years) for Xolairtreated patients and 5.12 (51/9,963 patient years) for control patients. In a multivariate analysis controlling for available baseline cardiovascular risk factors, the hazard ratio was 1.32 (95% confidence interval 0.91-1.91). In a new analysis of pooled clinical trials, which included all randomised doubleblind, placebo-controlled clinical trials lasting 8 or more weeks, the rate of ATE per 1,000 patient years was 2.69 (5/1,856 patient years) for Xolairtreated patients and 2.38 (4/1,680 patient years) for placebo patients (rate ratio 1.13, 95% confidence interval 0.24-5.71).
MHRA WARNING: Article date: February 2011
Omalizumab: potential risk of arterial thrombotic events
Summary: Use of omalizumab may be associated with an increased risk of arterial thrombotic events. Prescribers should be vigilant for possible thrombotic adverse reactions, and should report these events to us promptly via the Yellow Card Scheme
Arterial thrombotic events with omalizumab
In controlled clinical trials and an unpublished ongoing observational study (EXCELS), a numerical imbalance of arterial thrombotic events (ATEs) was observed in association with use of omalizumab; however, this finding was not statistically significant at the 95% level. ATEs included stroke, transient ischaemic attack, myocardial infarction, unstable angina, and cardiovascular death (including death from unknown cause).
Call for reporting : Prescribers should be vigilant for possible thrombotic adverse reactions. All suspected adverse reactions, including arterial thrombotic events, to
omalizumab▼ should be reported via the Yellow Card Scheme at www.yellowcard.gov.uk
Further information: BNF section 3.4.2 Allergen immunotherapy
The table below summarises interim data from EXCELS and data from controlled clinical trials:
|
|
Arterial thrombotic events per 1000 patient-years of treatment (patient years) |
Risk versus controls: hazard ratio, HR (95% CI) |
|
|
|
Omalizumab |
Controls |
|
|
EXCELS |
5.59 (79/14140) |
3.71 (31/8366) |
Adjusted* HR 1.11 (0.70– 1.76) |
|
|
Arterial thrombotic events per 1000 patient-years of treatment (patient years) |
Risk versus controls: hazard ratio, HR (95% CI) |
|
|
|
Omalizumab |
Controls |
|
|
Controlled clinical trials |
6.29 (17/2703) |
3.42 (6/1755) |
Unadjusted HR 1.86 (0.73–4.72) |
*Controlling for baseline cardiovascular risk factors
Platelets:In clinical trials few patients had platelet counts below the lower limit of the normal laboratory range. None of these changes were associated with bleeding episodes or a decrease in haemoglobin. No pattern of persistent decrease in platelet counts, as observed in non-human primates (see section 5.3), has been reported in humans (patients above 6 years of age), even though isolated cases of idiopathic thrombocytopenia have been reported in the post-marketing setting.
Parasitic infections: In patients at chronic high risk of helminth infection, a placebocontrolled trial showed a slight numerical increase in infection rate with omalizumab that was not statistically significant. The course, severity, and response to treatment of infections were unaltered.
IgE may be involved in the immunological response to some helminth infections. In patients at chronic high risk of helminth infection, a placebo-controlled trial showed a slight increase in infection rate with omalizumab, although the course, severity, and response to treatment of infection were unaltered. The helminth infection rate in the overall clinical programme, which was not designed to detect such infections, was less than 1 in 1,000 patients. However, caution may be warranted in patients at high risk of helminth infection, in particular when travelling to areas where helminthic infections are endemic. If patients do not respond to recommended anti-helminth treatment, discontinuation of Xolair should be considered.
- The ENFUMOSA Study Group. The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. Eur Respir J 2003;22(3):470-7.
- Hamelmann E. The rationale for treating allergic asthma with anti-IgE. Eur Respir Rev 2007;16:61-6.
- British guideline on the management of asthma. British Thoracic Society/Scottish Intercollegiate Guidelines Network. Thorax January 2016.
- GINA Report, Global Strategy for Asthma Management and Prevention Global Initiative for Asthma, 2018.
- Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007;120(5, Supplement 1):S94-S138.
- Walker S, Monteil M, Phelan K, Lasserson T, Walters E. Anti-IgE for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2014.
- Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and Safety of Subcutaneous Omalizumab vs Placebo as Add-on Therapy to Corticosteroids for Children and Adults With Asthma. Chest 2011;139(1):28-35.
- Corren J, Casale TB, Lanier B et al. Safety and tolerability of omalizumab. Clin Exp Allergy 2009;39(6):788-97.
- Aidan AL, James EF, Abdelkader R, et al. Baseline characteristics of patients enrolled in EXCELS: a cohort study. Ann Allergy Asthma Immunol 2009;103(3):212-9.
Last reviewed: 31 January 2016
Next review: 31 January 2019
Author(s): West of Scotland Difficult Asthma Group
Approved By: West of Scotland Difficult Asthma Group
Reviewer Name(s): Isobel Baxter, Respiratory MCN Coordinator