Scottish Paediatric Consensus Treatment Guideline for Paediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2 (PIMS-TS)
exp date isn't null, but text field is
Objectives
This guideline summarises case definitions, referral pathway, and treatment options for children in Scotland admitted to hospital with symptoms consistent with Paediatric Inflammatory Multisystem Syndrome temporally associated with the SARS-CoV-2 pandemic (PIMS-TS). It also provides definitions and referral pathways of complete and incomplete Kawasaki Disease (KD) as the primary differential diagnoses of PIMS-TS but is not intended to provide guidance on the management of KD which should follow standard practice. The incidence of PIMS-TS is currently very low, but this may change in future if new variants of SARS-CoV-2 emerge or if there is reduction in population immunity. Given the current rarity of PIMS-TS, alternative diagnoses should be carefully considered.
Scope
This is a national consensus guidance document for paediatric units in Scotland produced by the Scottish Hyperinflammatory Multidisciplinary Team (MDT). It is informed by and designed to complement guidance produced by the RCPCH. Some additional guidance has been added based on the UK Delphi process[1]. Guidance will be updated as further evidence arises about best practice in the management of PIMS-TS and related conditions. It is not intended as guidance for primary care. Children with symptoms suggestive of PIMS-TS or KD, should always be referred acutely to secondary care. Separate guidance is available for the treatment of acute Covid-19.
1.1 Kawasaki Disease (KD) Criteria
“Complete” KD is defined according to American Heart Association[2] criteria as :
Fever of at least five days in addition to 4 of 5 additional criteria:
- Conjunctivitis: bilateral, bulbar, conjunctival injection without exudate
- Lymphadenopathy: cervical, often >1.5cm, usually unilateral
- Rash: maculopapular, diffuse erythroderma or erythema multiforme
- Changes of lips or oral mucosa: red cracked lips, “strawberry” tongue, or diffuse erythema of oropharynx
- Changes of extremities: erythema, and oedema of palms and soles in acute phase; and periungual desquamation in subacute phase.
OR less than 5 days of fever but otherwise meeting all five AHA criteria.
OR less than 5 days of fever with coronary artery aneurysm or coronary dilatation.
1.2 Incomplete KD Criteria
Children (>1 year old) with fever for ≥5 days AND at least 2 other compatible clinical criteria listed above; OR infants ≤ 1year with fever ≥7 days without other explanation AND (for both age groups) CRP≥30 mg/L or ESR ≥40 mm/h AND
- EITHER the presence of any 3 or more of: anaemia for age (haemoglobin less than the lower limit of normal laboratory reference range for age); platelet count ≥ 450 x109/L or <140 x109/L; albumin < 30g/L; elevated ALT, WCC <15x 109/L; urine ≥10 WBC per high power field
- OR abnormal echocardiogram compatible with KD but without established CAA, with ≥3 of the following suggestive features: decreased left ventricular function, mitral regurgitation, pericardial effusion, or dilated but non-aneurysmal coronary arteries
1.3 Paediatric Inflammatory Multisystem Syndrome Temporally associated with SARS-CoV-2 (PIMS-TS)
RCPCH case definition:
- A child presenting with persistent fever, inflammation (neutrophilia, elevated CRP and lymphopenia) and evidence of single or multi-organ dysfunction (shock, cardiac, respiratory, renal gastrointestinal or neurological disorder) with additional features (see below). This may include children fulfilling full or partial criteria for Kawasaki disease.
- Exclusion of any other microbial cause, including bacterial sepsis, staphylococcal or streptococcal shock syndromes, infections associated with myocarditis such as enterovirus (waiting for results of these investigations should not delay seeking expert advice).
- SARS-Cov-2 PCR testing may be positive or negative.
Additional Features in PIMS-TS
Clinical Features:
- ALL: Persistent fever >38.5°C
- MOST: Oxygen requirement or hypotension
- SOME: abdominal pain, confusion, conjunctivitis, cough, diarrhoea, headache, lymphadenopathy, mucus membrane changes, neck swelling, rash, resp symptoms, sore throat, swollen hands and feet, syncope, vomiting
Laboratory
- ALL: abnormal fibrinogen, absence of potential causative organisms (other than SARS-CoV-2), high CRP, high D-Dimers, high ferritin, hypoalbuminaemia, lymphopenia
- Neutrophilia in most– normal neutrophils in some
- SOME: acute kidney injury, anaemia, coagulopathy, proteinuria, raised CK, raised LDH, raised triglycerides, raised troponin, thrombocytopenia, transaminitis
Imaging and ECG
- Echo and ECG – myocarditis, valvulitis, pericardial effusion, coronary artery dilatation
- CXR – patchy symmetrical infiltrates, pleural effusion
- Abdo USS – colitis, ileitis, lymphadenopathy, ascites, hepatosplenomegaly
- CT chest – as for CXR – may demonstrate coronary artery abnormalities if with contrast
Note: WHO refer to PIMS-TS as Multisystem Inflammatory Syndrome in Children (MIS-C). The WHO case definition is similar, but requires at least 3 days of fever and either evidence of COVID-19 on PCR or serology or a likely contact with COVID-19.
Case definitions are preliminary and subject to change. Check for updates here:
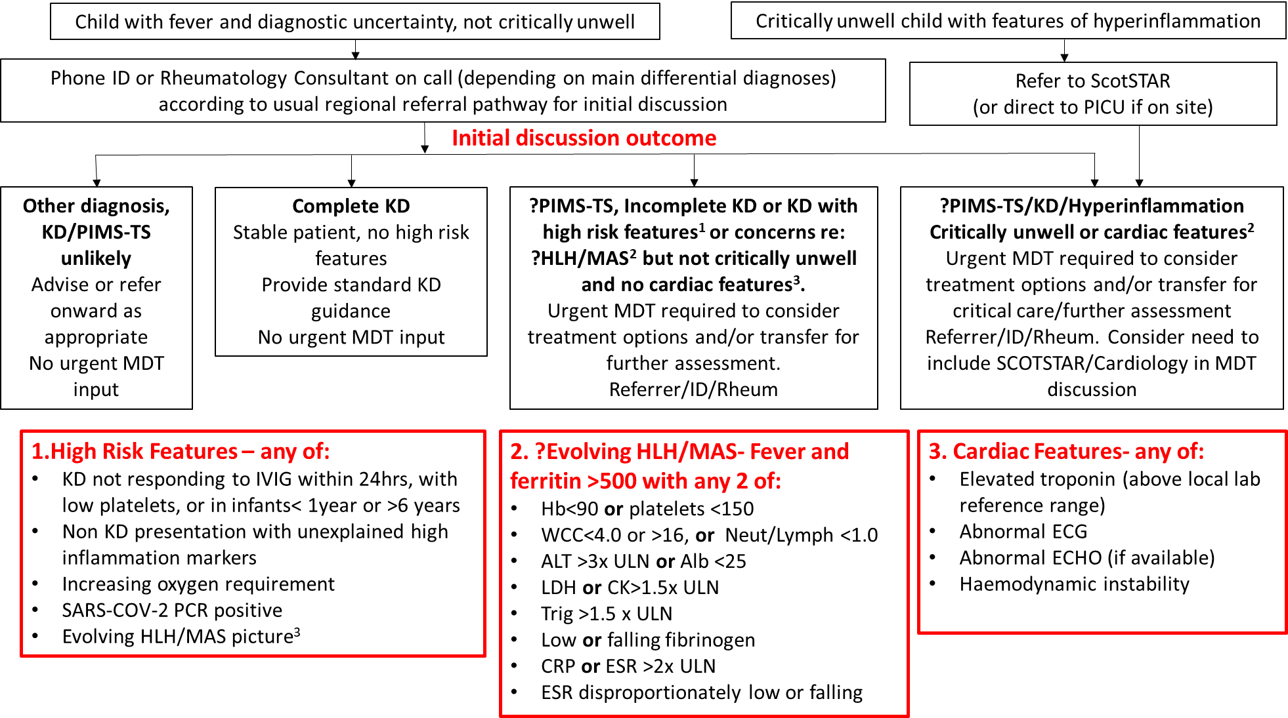
Figure 1: Referral Pathway for possible Hyperinflammatory syndrome
This guidance outlines the referral pathway for children with signs/symptoms consistent with hyper-inflammatory conditions or PIMS-TS to the Hyperinflammatory Multi-disciplinary Team at the Royal Hospital for Children, Glasgow or Royal Hospital for Sick Children, Edinburgh from paediatric units throughout Scotland. Patients presenting with hyper-inflammatory conditions can be complex; there may be diagnostic difficulties and management can be challenging. These conditions are rare, there is limited trial data to support management decisions and therefore local MDT discussion about diagnostics and management is beneficial. With the recent emergence of a cohort of children, presenting acutely unwell with an inflammatory multisystem syndrome, with some features similar to Kawasaki disease, some similar to toxic shock syndrome and some with features in keeping with macrophage activation syndrome (MAS)/haemophagocytic lymphohistiocytosis (HLH), there is a need for multi-disciplinary team working to manage these children.
MDT referral or discussion should be considered for children presenting with features consistent with any of the case definitions above. Children do not need to fulfil the full case definition to be referred/discussed and discussion about children with pyrexia of unknown origin, Kawasaki disease or evidence of hyper-inflammation without clear cause is encouraged. The urgency of referral, and the members of MDT required for discussion should be determined by clinical status as follows:
2.1 Critically unwell children or children with cardiac features
In paediatric units without PICU on site, where the child is critically unwell and thought to need ScotSTAR transfer the referral should be through the usual referral pathway to ScotSTAR (03333990222) who will then involve the wider MDT at an appropriate time. ScotSTAR will then decide if the child should be transferred by ScotSTAR or the Scottish Ambulance Service (SAS).
In paediatric units with PICU on site, direct referral to the PICU on call team should be made as per usual pathways and wider MDT discussion can be convened at an appropriate time.
Referral to PICU/ ScotSTAR should be considered when there is:
- Need for critical care support including: escalating non-invasive respiratory support above that provided comfortably by referring ward/hospital, invasive respiratory support and/or inotropic support or advanced support e.g. renal support or extra-corporeal support (ECMO).
- Cardiovascular shock not reversed by standard fluid resuscitation.
- Any deteriorating patient where there is clinical concern that would normally trigger a discussion with PICU.
- Significant cardiac findings on echocardiogram thought to increase the risk of acute deterioration for the patient.
- Patients requiring transfer to RHC Glasgow or RHSC Edinburgh out with the above criteria where there is significant concern regarding safe transfer due to likely patient deterioration.
In children with raised troponin (above local lab reference range), abnormal ECG, abnormal ECHO or haemodynamic instability, discussion with the Paediatric Cardiology Consultant on call should also be considered. At the point of referral to cardiology, desired tests include CXR for evidence of cardiomegaly, Echocardiogram (if available), troponin, NT-pro BNP (if available), but this should not delay MDT discussion. Both RHSC Edinburgh and RHC Glasgow have cardiology services, including cardiologist input and echocardiography. Patients requiring specialist cardiac or cardiac ICU services (including ECMO) should be discussed with Glasgow if potential transport is required.
Children with possible hyperinflammatory syndromes referred firstly to PICU/Scotstar should be discussed with the wider MDT as soon as this felt to be appropriate by the PICU/Scotstar team to discuss early treatment options. Specific treatments (for example steroids or IVIG) may be appropriate prior to transfer, and will be considered by the MDT on a case-by-case basis.
2.1.1 Guidance on Cardiology Investigations and Management
- 12 lead ECG, echocardiogram, Troponin and NT-proBNP should be performed on admission for all PIMS-TS patients.
- If clinically suspected myocarditis, initiate Myocarditis investigations per protocol https://www.clinicalguidelines.scot.nhs.uk/ggc-paediatric-guidelines/ggc-guidelines/cardiovascular-diseases/cardiomyopathy-and-myocarditis-investigations-protocol/
- Haemodynamically unstable patients will be managed on PICU and have daily 12 lead ECG, daily echocardiograms and 12-24 hourly cardiac biomarkers (cTn and NT-pro-BNP)22. Low threshold for Milrinone infusion, consider Levosimendan or pressor agents, VA ECMO for refractory shock.
- Haemodynamically stable patients will have a minimum of 2 inpatient ECG, 2 echocardiograms and 2 sets of cTn and NT-proBNP whilst inpatient. Consider continuous ECG monitoring during first 24-48 hours after admission
- 24 hour ambulatory ECG recording is required if tachyarrhythmias or evidence of any degree of AV block (to be agreed/requested by Cardiology)
2.2 Initial referral discussions for children who are not critically unwell
Initial referral should be by telephone to the on-call consultant for either Infectious Diseases (ID) or Rheumatology via switchboard at RHC, Glasgow or RHSC, Edinburgh according to usual regional referral pathways. Broadly speaking, initial discussions should be with ID if differential diagnoses are thought to mainly include acute COVID-19 or other infections, or with Rheumatology if differential diagnoses are thought to mainly include systemic rheumatological or inflammatory conditions. However, given the overlap in differential diagnoses either service welcomes initial discussion.
Following this initial discussion, if there are any high-risk features (detailed below), or if agreed by the referring and receiving consultant, a wider MDT is required, as per the flowchart above. This MDT will include the referring consultant, ID and Rheumatology, and may include PICU/Scotstar or cardiology as per guidance above.
High risk features which should prompt a wider urgent MDT include:
- Kawasaki disease (KD) not responding to IVIG within 24hrs, with low platelets, or in infants< 1year or >6 years
- Non KD presentation with unexplained high inflammation markers
- Increasing oxygen requirement
- SARS-COV-2 PCR positive
- Evolving HLH/MAS picture, defined as fever and ferritin>500 with any 2 of:
- Hb<90 or platelets <150
- WCC <4.9 or >16, or Neut/Lymph <1.0
- ALT >3x upper limit of normal (ULN) or Albumin<25
- LDH or CK >1.5x ULN
- Triglyceride >1.5x ULN
- Low or falling fibrinogen
- CRP or ESR >2x ULN
- ESR disproportionately low or falling
The MDT discussion will be arranged via Microsoft teams by the consultant receiving the initial referral, or via Scotstar if the child is critically unwell.
Children who are stable and require transfer for specialty input/investigation do not require ScotSTAR and should be transferred by SAS.
Treatment options for KD and PIMS-TS are summarised in Figure 2. For PIMS-TS and Incomplete KD in the context of the SARS-CoV-2 pandemic, treatment should be discussed with the Scottish Hyperinflammatory MDT (see details for referral pathway above).
Figure 2: Treatment algorithm for KD and PIMS-TS
The options by age and clinical presentation are as follows:
3.1 “Complete” Kawasaki disease
Children who meet full criteria for KD, as defined above, should be treated in accordance with standard KD guidelines with IVIG 2g/kg, high dose aspirin, and cardiac follow up. Children with KD admitted to RHC, Glasgow may be considered for the KD-CAAP trial (which randomises to IVIG with or without early steroid treatment).
Consideration may be given to steroids, repeat IVIG or biologic treatments in certain high risk groups or in children failing to respond to initial treatment (https://adc.bmj.com/content/99/1/74)[3].
3.2 Incomplete Kawasaki Disease
Children who meet AHA criteria for incomplete KD as defined above should be treated with IVIG, high-dose aspirin and cardiac follow-up in accordance with standard KD guidelines. Given the overlap with non-specific PIMS-TS and other conditions, children with Incomplete KD should ideally be discussed at MDT to agree that criteria for this diagnosis are met. In Glasgow, they should be considered for the KD-CAAP trial.
3.3 Non-specific PIMS-TS
Given the overlap with other conditions, children with suspected non-specific PIMS-TS should be discussed at MDT to agree the diagnosis and treatment plan. First line treatment in severe/critical disease would be methylprednisolone. Children should be given empiric broad spectrum antibiotic cover with ceftriaxone until severe bacterial infection can be excluded. Children with features of toxic shock syndrome should also be given high dose clindamycin. IV fluid boluses should be used in increments with close assessment of response given the risk of cardiac complications. Compression stockings and low molecular weight heparin (LMWH) prophylaxis should be considered in severely unwell children over 12 who are immobile or have other risk factors for thrombosis. Low-dose aspirin should be given to all children with a diagnosis of PIMS-TS for at least 6 weeks.
In children failing to improve after 24 hours or who are rapidly clinically deteriorating despite steroids, give IVIG. Biological treatment should be considered as third line in children not responding to treatment with IVIG and steroids, and the decision to give a biological treatment should be made by the MDT.
|
Drug for treatment of non-specific PIMS-TS |
Route of administration |
Dose |
Treatment duration |
|
Methylprednisolone |
IV |
2mg/kg for children without shock 10mg-30mg/kg once daily (max 1g) for children with shock. 30mg/kg can be considered in children with suspected HLH/MAS.
|
3 days |
|
Immunoglobulin |
IV |
2g/kg once only. For obese children use weight at 91st centile for age. Automatic approval under National IVIg demand programme. Can be repeated if not improving after 48 hours after discussion with MDT |
Single dose |
|
Tocilizumab |
IV |
2nd line option in children >1 year old <30kg 12mg/kg single dose ≥30kg 8mg/kg single dose (max 800mg) A second dose may be given ≥12 and ≤24 hours later if the patient’s condition has not improved. |
1-2 doses only |
|
Aspirin |
oral |
Low dose 2-5mg/kg once daily |
For minimum 6 weeks |
|
Anakinra |
Sc/IV bolus |
2nd line option in children >1year and >10kg: Start at 2mg/kg OD (max 100mg), increasing in increments of 2mg/kg/day if unresponsive. Max 8mg/kg (400mg)/day |
7 days |
Additional notes on Anakinra:
The doses above are based on the PIMS-TS arm of RECOVERY.
Anakinra may be given as a continuous IV infusion if the child is critically unwell and there is concern about systemic absorption. This would usually be in the intensive care setting. There are no established dose ranges for children. A loading dose of 2mg/kg (max 100mg) is given followed by continuous infusion starting at 2mg/kg/day but can be increased under specialist advice. Anakinra has been shown to be safe and does not increase mortality in patients with sepsis.
3.4 Special considerations in treatment dosing
3.4.1 Obesity
In children with severe obesity, dosing of IVIG should be based on the 91st centile weight for age from the WHO/RCPCH growth centile chart, rather than actual body weight. For other drugs, care should be taken to adhere to maximum dosing.
3.4.2 Renal and Hepatic Impairment
Aspirin should be used with caution in mild-moderate renal and hepatic impairment, and avoided in severe impairment. Tocilizumab should be used with caution in renal and hepatic impairment, and renal function should be monitored closely. Remdesivir is contraindicated in GFR <30ml/min/1.73m2 and where ALT/AST >5 x ULN for age.
Where renal or liver impairment is identified, treatment should be discussed at MDT with input from a specialist paediatric pharmacist, and a risk vs benefit decision agreed.
4.1 General Follow-up
Discharge may be considered when children have been afebrile for at least 24 hours and have stable cardiac function. Children with KD and Incomplete KD should have follow-up as per standard guidelines. As a minimum, children with PIMS-TS should have follow-up 1-2 weeks post discharge, and further follow-up 6 weeks after discharge.
4.2 Cardiology follow-up
Children with KD and incomplete KD should have cardiology follow-up as per standard guidelines.
For patients who presented with PIMS-TS and features of pancarditis (biventricular function impairment, mitral/tricuspid valve regurgitation, diastolic dysfunction, pericardial effusion, coronary artery dilatation/aneurysm), consider cardiology follow up
- at 1-2 weeks post-discharge
- at 2, 4 and 6 weeks, or until all investigations results normalise
- consider early outpatient cross-sectional imaging (CT/MRI).
All other children with PIMS-TS should have cardiology follow-up at 6 weeks post discharge as a minimum. Low-dose aspirin should be continued until 6 week follow-up. Longer term antiplatelet/anticoagulation in children with coronary artery abnormalities should be discussed with cardiology/haematology.
All referred cases of PIMS-TS and complex cases of KD will be discussed for the purposes of peer review and sharing experiences at the Scottish Hyperinflammatory Syndrome MDT meeting. This is currently held quarterly, but may be held more frequently if case number rise. To join the mailing list for this meeting, or to suggest cases or topics for discussion, please email mary.glen@nhs.scot
The ISARIC clinical characterisation study is now closed for Covid-19. The BPSU survey is now closed for PIMS-TS. The RECOVERY trial randomisation for PIMS-TS is now closed.
The KD-CAAP trial is a European multicentre, randomised, open-label, blinded endpoint assessed trial of corticosteroids plus IVIG and aspirin, versus IVIG and aspirin for prevention of coronary artery aneurysm in Kawasaki disease. In Scotland, this trial is currently only recruiting in Glasgow, as there were limits to the number of centres within a single country which could recruit. Aberdeen is a reserve centre and may recruit at a later date. Children from other centres should not be transferred to Glasgow for the sole purpose of entering this trial, but if transferred there for other reasons may be eligible. Patients who may be eligible for KD-CAAP in Glasgow should be discussed with the ID or Rheumatology consultant on call.
The Consultant on call for Rheumatology or Infectious Diseases at the Royal Hospital for Children, Glasgow can be reached through hospital switchboard (0141 201 0000) 24 hours a day.
- Harwood R, Allin B, Jones CE, et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc Health 2020.
- McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017; 135(17): e927-e99.
- Eleftheriou D, Levin M, Shingadia D, Tulloh R, Klein NJ, Brogan PA. Management of Kawasaki disease. Arch Dis Child 2014; 99(1): 74-83.
Last reviewed: 13 July 2022
Next review: 31 July 2025
Author(s): Dr Louisa Pollock
Co-Author(s): Dr Kirsty McLellan; Susan Kafka
Approved By: Scottish Hyperinflammatory MDT
Document Id: 1104