Scottish Paediatric Treatment Guideline for Children in Hospital with Acute Covid-19
exp date isn't null, but text field is
Objectives
This guideline summarises case definitions and treatment options in hospital for children in Scotland with acute Covid-19. This includes both children hospitalised due to Covid-19, and children who are SARS-CoV-2 positive and who are hospitalised for indications other than the management of acute symptoms of COVID-19 (hospital onset/incidental Covid-19).
The pathway for management and referral of children with symptoms consistent with a diagnosis of Paediatric Inflammatory Multisystem Disorder Temporally Associated with SARS-CoV-2 (PIMS-TS) and similar hyperinflammatory disorders is covered in separate guidance (PIMS-TS guideline).
The pathway for management and referral of non-hospitalised children with SARS-CoV-2 at high risk of Covid-19 is covered in separate guidance (Non-hospitalised children with SARS-CoV-2 guideline).
Scope
This is a national consensus guidance document for paediatric units in Scotland produced by the Scottish Paediatric & Adolescent Infection & Immunology National Managed Clinical Network (SPAIIN). It is informed by and designed to complement guidance produced by the RCPCH1, NICE2, WHO3 and UK Commissioning bodies4. Guidance will be updated as further evidence arises about best practice in the management of paediatric Covid-19 and related conditions. It is not intended as guidance for primary care.
Symptoms in children have varied with the circulating strain, but commonly include fever, upper and lower respiratory tract symptoms (sore throat, runny nose, cough), gastrointestinal symptoms, loss of sense of taste or smell, headache, myalgia, fatigue and rash.5 Presentation in children is non-specific and very similar to other febrile childhood illness. A high proportion of children with SARS-CoV-2 have asymptomatic infection, therefore in some PCR/point of care test positive children, symptoms may be due to other infections or diseases. There may be some overlap in clinical features of acute Covid-19 and PIMS-TS/KD. For guidance on management and referral pathways for PIMS-TS please see separate guidance.
Comorbidities which place children in the highest risk group for progression to severe Covid-19 are summarised in Table 1. These risk factors are defined in government guidance here and are identical to RCPCH guidance.1
Table 1: RCPCH risk factors for progression to severe Covid-19
|
Children and young people (CYP) at substantial risk |
|
|
CYP at significant risk if 2 or more of these risk factors are present |
|
Primary immunodeficiency:
|
|
Secondary immunodeficiency:
|
|
Immunosuppressive treatment:
|
|
Other conditions:
|
The UK Interim Clinical Commissioning policy refers to a definition of “hospital onset” acute Covid-19, for patients who were hospitalised for indications other than for the management of acute symptoms of Covid-19, who then develop symptomatic SARS-CoV-2 infection. A similar approach may be taken for children who are admitted for other indications and found to be SARS-CoV-2 positive on admission screening. A recent UK study found that a positive SARS-CoV-2 test was an incidental finding and not the reason for admission in at least 20% of hospitalised children with confirmed SARS-CoV-2.5
There are no current recommendations for treating hospitalised children with asymptomatic SARS-CoV-2 infection, even in those at highest risk of progression to severe disease. A careful “watch and wait” approach with a low threshold for early treatment with symptom onset is appropriate.
The UK Interim Clinical Commissioning Policy (update November 2022)6 recommends treatment may be considered in children who were hospitalised for indications other than for the management of acute symptoms of Covid-19 who meet the following criteria:
- Symptomatic with COVID-19 and showing no signs of clinical recovery
- AND A member of the ‘highest risk’ group as defined by paediatric MDT assessment (in practice this is undertaken using the RCPCH (COVID management guidelines) risk criteria)
OR - COVID-19 presents a material risk of destabilising a pre-existing condition or illness or compromising recovery from surgery or other hospital procedure.
Treatment options include remdesivir and nirmatrelvir/ritonavir (Paxlovid). Note, Paxlovid is currently unlicensed in children under 18 years in the UK and has multiple drug interactions. Remdesivir is licensed for treatment of children weighing >40kg who do not require supplemental oxygen who are at high risk of progression to severe disease. There are therefore no licensed anti-viral treatment options for children weighing under 40kg for this indication, but off-license treatment may be agreed on a case by case basis.
National treatment guidelines for children are currently subject to review. Treatment should always be discussed with the paediatric infectious diseases team.
Children who deteriorate and develop severe or critical disease should be managed as per guidance below.
Recommended treatments for children hospitalised due to Covid-19 are based on disease severity.
NICE2 and WHO3 define severity of COVID-19 as follows:
Critical COVID-19 – Defined by the criteria for acute respiratory distress syndrome (ARDS), sepsis, septic shock, or other conditions that would normally require the provision of life-sustaining therapies such as mechanical ventilation (invasive or non-invasive) or vasopressor therapy.
Severe COVID-19 – Defined by any of:
- oxygen saturation < 90% on room air;
- signs of pneumonia;
- signs of severe respiratory distress (in adults, accessory muscle use, inability to complete full sentences, respiratory rate > 30 breaths per minute; and, in children, very severe chest wall in-drawing, grunting, central cyanosis, or presence of any other general danger signs including inability to breastfeed or drink, lethargy, convulsions or reduced level of consciousness).
Non-severe COVID-19 – Defined as the absence of any criteria for severe or critical COVID-19.
NICE treatment guidelines for Covid-19 are currently under review. Further guidance may be available through Covid-19 Alerts . Treatment should always be discussed with the paediatric infectious diseases team.
Treatment options for non-severe Covid-19
- No treatment is advised in children with non-severe Covid-19 who are not at high risk of severe disease.
- For children at high risk, a similar approach to that recommended for hospital onset/incidental SARS-CoV-2 in high-risk patients, including use of remdesivir, may be considered. This should be discussed with the paediatric infectious diseases team.
Treatment options for severe and critical Covid-19
Treatment options by age for severe and critical Covid-19 are as follows:
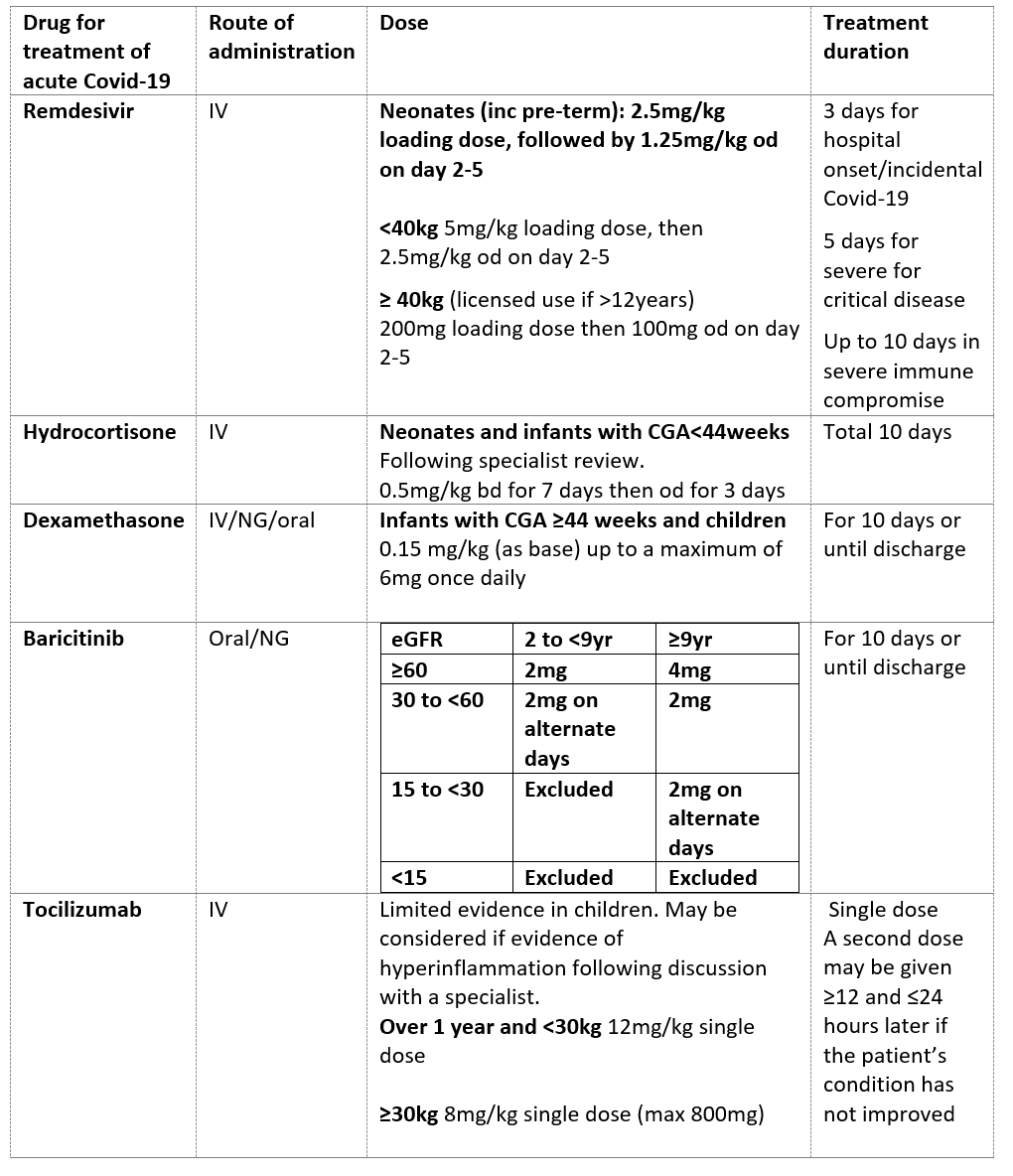
Dexamethasone
- Dexamethasone is now standard of care in children over 5 years of age with severe or critical COVID-19 as defined by WHO/NICE.2,3
- For infants over 44 weeks corrected gestational age to 5 years, dexamethasone can be considered in discussion with the paediatric infectious diseases team.
- For neonates and preterm infants <44 weeks corrected gestational age hydrocortisone may be considered following discussion with the paediatric infectious diseases team.
- Dexamethasone should be continued for 10 days but can be stopped early if the child improves and is discharged.
Baricitinib
Baricitinib (a selective and reversible Janus kinase (JAK) 1 and 2 inhibitor) has shown benefit in reducing mortality in adults with severe respiratory Covid-19. It has previously been used in children in the treatment of rheumatoid arthritis and other inflammatory conditions and works best in Covid-19 where there are signs of inflammation. Children over 2 years are eligible to receive Baricitinib7 if all the following criteria are met:
- Confirmed or high clinical suspicion of Covid-19
- Viral pneumonia syndrome present and receiving supplemental oxygen or respiratory support
- Receiving dexamethasone unless contra-indicated
- No known hypersensitivity to Baricitinib
- eGFR>15 if over 9 years old, or >30 if <9 years old
- not receiving dialysis or haemofiltration
- absolute neutrophil count >0.5 x109 cells/L
- no active tuberculosis
- not pregnant or breastfeeding
Treatment with baricitinib should be discussed with the paediatric infectious diseases team.
Remdesivir
Remdesivir is currently the only anti-viral therapy for SARS-CoV-2 that is licensed for use in children (over 4 weeks of age and weight ≥3kg), with acute respiratory Covid-19 in oxygen. It is a direct acting anti-viral which works best in early disease. It is safe and well tolerated. Evidence of benefit of remdesivir in the treatment of acute COVID requiring hospitalisation is limited. A course of remdesivir (up to 5 days) can be considered in patients who meet all of the following criteria:
- Confirmed or high clinical suspicion of Covid-19
- Hospitalised specifically for the management of Covid-19
- Requiring low-flow supplemental oxygen
- Presented to hospital not more than 10 days since symptom onset
- eGFR >30
- ALT <5 times the upper limit of normal
Exemptions to the criteria on symptom onset and supplemental oxygen can be made for immunocompromised patients.
Remdesivir should be discontinued in patients who develop any of the following:
- ALT≥5 times the upper limit of normal
- ALT elevation accompanied by signs or symptoms of liver inflammatio0n or increasing conjugated bilirubin, ALP or INR.
Treatment should be discussed with the paediatric infectious diseases team.
Immunomodulatory treatments
For immunomodulatory therapies other than dexamethasone and Baricitinib, there is limited published evidence of efficacy of specific therapies for COVID -19 in adults, and a lack of high-quality evidence in children. UK Clinical Commissioning/NICE recommendations for Tocilizumab currently only apply to adults.2,8 Sarilumab is no longer recommended by NICE.2
Immunomodulatory therapy may only be indicated if clear evidence of hyperinflammation, or in the second phase of the illness. Treatment should be discussed with the paediatric infectious diseases team.
Prevention of thrombosis
Compression stockings and low molecular weight heparin (LMWH) prophylaxis should be considered in severely unwell children over 12 who are immobile or have other risk factors for thrombosis.
Bacterial Co-infection
If bacterial co-infection is clinically suspected local empiric antibiotic guidelines should be followed.
Renal and liver function should be checked prior to initiation of therapy and medication adjusted where required. Discuss with Pharmacy. Check for drug-drug interactions at https://www.covid19-druginteractions.org/ or contact Pharmacy for advice.
Table 2: Paediatric Drug Treatments for Acute Covid-19
The ISARIC clinical characterisation study is now closed for Covid-19.
The RECOVERY trial is the largest collaborative UK trial of treatment for Covid-19. There are no active treatment arms for children at present. Details of the RECOVERY trial can be found here.
Royal Hospital for Children, Glasgow:
The Paediatric Infectious diseases team can be reached Mon-Fri 9-5pm via 84939 (outside calls 0141 452 4939). Outwith these hours the Consultant on call for Paediatric Infectious Diseases should be contacted via switchboard (0141 201 0000).
- Royal College of Paediatrics and Child Health. COVID-19 - guidance for management of children admitted to hospital and for treatment of non-hospitalised children at risk of severe disease.
- National Institute for Clinical Excellence. COVID-19 rapid guideline: managing COVID-19. Nice guideline NG191.
- Agarwal A, Rochwerg B, Lamontagne F, Siemieniuk R A, Agoritsas T, Askie L et al. A living WHO guideline on drugs for covid-19 BMJ 2020; 370 :m3379 doi:10.1136/bmj.m3379
- Medicines and Healthcare products Regulatory Agency: Coronavirus (Covid-19) Alerts and Registration
- Swann, O.V., Pollock, L., Holden, K.A. et al. Comparison of UK paediatric SARS-CoV-2 admissions across the first and second pandemic waves. Pediatr Res (2022).
- Medicines and Healthcare products Regulatory Agency: Coronavirus (Covid-19) Alerts: Antivirals or Neutralising Monoclonal Antibodies in the Treatment of Hospital-Onset COVID-19. 28th November 2022
- Medicines and Healthcare products Regulatory Agency: Coronavirus (Covid-19) Alerts: Baricitinib for Patients Hospitalised Due to COVID-19 (Adults and Children Aged 2 Years and Over)
- Medicines and Healthcare products Regulatory Agency: Coronavirus (Covid-19) Alerts: Interleukin-6 inhibitors (tocilizumab or sarilumab) for adult patients hospitalised due to COVID-19
Last reviewed: 12 September 2023
Next review: 30 September 2026
Author(s): Louisa Pollock
Author Email(s): louisa.pollock@ggc.scot.nhs.uk
Co-Author(s): Susan Kafka
Approved By: Paediatric Guidelines Group
Document Id: 1112