Total Intravenous Anaesthesia (TIVA) : a guide to using propofol and remifentanil mixed in the same syringe
exp date isn't null, but text field is
Objectives
This guide overviews how to deliver general anaesthesia using a propofol / remifentanil mixture, including for MLB and GI endoscopy.
Safe Use of Total Intravenous Anaesthesia (TIVA)
There are safety implications when using TIVA. The Association of Anaesthetists published their guideline “Safe Practice of Total Intravenous Anaesthesia” in 2018 [1]. Below is an adaptation of the key safety recommendations made by the Association of Anaesthestists, highlighting those of particular relevance in our institution. These should be read in conjunction with the full guideline [1].
Recommendations
- Anaesthetists should ensure they have the knowledge and skills required to deliver TIVA competently and safely, forming part of their ongoing career-long learning.
- For propofol delivery, only target-controlled infusion (TCI) pumps should be used, and should have the appropriate TCI programmes installed.
- Infusion pumps being used should be plugged in and charged before use. Where possible, they should be continuously plugged in during use.
- Infusion pumps should only be programmed after the filled & labelled drug syringe has been inserted. This reduces risk of incorrect programming, a cause of accidental awareness under general anaesthesia (AAGA).
- Infusion pumps should be visible at all times when in use, and should be checked every few minutes for infusion rate, correct settings and drug volume remaining.
- Intravenous giving sets should meet all safety requirements for TIVA use. *In RHC Glasgow, Mediplus TIVA giving sets meet all the requirements and should be the only giving sets used for TIVA.
- Intravenous cannulae being used for TIVA must be secure throughout use to avoid displacement and, whenever practical, should be visible at all times. This may involve the use of clear surgical drapes.
- Luer-lock syringes must be used to reduce the risk of disconnection. In RHC Glasgow, Alaris PK infusion pumps are programmed to accept B. Braun Omnifix 50ml syringes.
- Syringes being used should be correctly labelled with the name and concentration of drug.
- TIVA must be titrated to clinical parameters. The plasma and effect-site concentrations displayed cannot be assumed to be accurate due to inter-patient pharmacological variability.
- Neuromuscular blocking agents (NMBA) must only be administered to patients once loss of consciousness has occurred.
- Processed EEG (pEEG) monitoring is recommended when a NMBA is used with TIVA. The large majority of cases of self-reported AAGA in NAP5 occurred in patients who had received a NMBA. Processed EEG monitoring should ideally start before administration of a NMBA.
- Care must be taken to deliver adequate anaesthesia during the change from volatile anaesthesia to TIVA. Too low a starting Cpt may lead to AAGA, especially with concurrent use of a NMBA.
- All vascular access devices used for anaesthesia should be flushed with at least twice the dead space volume of the device at the end of the procedure. Ensure that the flush volume is sufficient to remove trace of drugs from the hub as well as the main lumen.
- When TIVA is used outside the operating theatre, for example during transfer, the same standards of monitoring should be maintained whenever possible, including pEEG monitoring if neuromuscular blockade is being used.
- Although rare, anaesthetists should always be aware of the risk of Propofol-Related Infusion Syndrome (PRIS). [see appendix]
TIVA in children can be delivered in different ways. For short to medium length cases, anaesthesia can be achieved by mixing propofol and remifentanil in the same syringe. The Association of Anaesthetists states that “mixing of propofol and remifentanil in the same syringe is not recommended”.
Bagshaw et al [2] studied nearly 900 anaesthetics in the UK to determine the safety profile the propofol / remifentanil mixture. The complication rate was found to be comparable to other anaesthetic techniques, with no complications related specifically to the mixing of drugs.
This guide overviews how to deliver general anaesthesia using a propofol / remifentanil mixture, including for MLB and GI endoscopy.
Separate propofol and remifentanil infusions are preferred in e.g. longer cases, cases needing more precise titration of drugs (neurosurgery) and cases where movement would be undesirable (middle ear surgery). With mixing of drugs, over time, the propofol TCI programme will reduce the rate of drug administration to maintain effect site concentration, but this will lead to a relative decrease in remifentanil administration rate, leading to inadequate analgesia which, in turn, can lead to inadequate depth of anaesthesia.
Giving Sets
There must be a luer-lock connector at each end, an anti-syphon valve on the drug delivery line and an anti-reflux valve on any fluid administration line. [1]
RHC Glasgow stocks Mediplus TIVA sets, which fulfil these requirements:
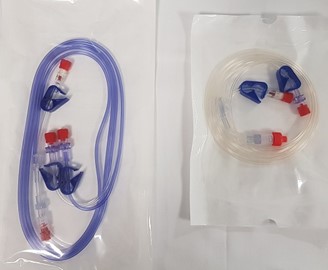 Figure 1. Mediplus giving sets for TIVA
Figure 1. Mediplus giving sets for TIVA
(left) 3-way TIVA set, 2.0m. Used when propofol and remifentanil are to be administered from separate syringes
(right) 2-way IV set, 2.0m. Used when propofol and remifentanil are to be administered from the same syringe
Alaris PK Infusion Pump (not Alaris CC)
Only the Alaris PK infusion pump is capable of TCI programs.
Figure 2. Alaris PK infusion pump

TCI Program
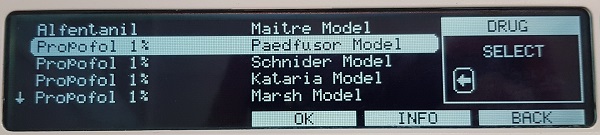
The “Propofol 1% Paedfusor Model” should be selected. The effect site concentration Ce is not calculated with the Paedfusor model, due to the variability of pharmacokinetics in children. Only the Cpt (target plasma concentration) and the Cp (calculated plasma concentration) are displayed. On changing the Cpt, there will be a delay of at least 30 to 60 seconds before the displayed Cp can be assumed to be the “true” Ce.
For adolescents the Marsh model may more appropriate due to a more “adult” metabolism. Also note the maximum programmable weight for the Paedfusor programme is 61kg.
Figure 3. Screen showing selection of Propofol 1% Paedfusor Model
Propofol / Remifentanil Mixture
It is recommended only to use 1% propofol because 2% propofol is associated with increased pain at the site of infusion. For long cases 2% propofol is appropriate but in these cases the drugs would be delivered via separate infusions so 2% propofol is not in the remit of this guide.
The use of a small volume of lidocaine reduces the incidence of pain at induction. This can be added to the pump or directly to the vein after cannulation. Without lidocaine, the incidence of pain at induction is unsatisfactorily high.
There are variations in practice regarding the final concentration of remifentanil in the propofol/remifentanil mixture. This is dependent on both the operator and the procedure being carried out. See tables 1 to 4 at the end of the document for how to achieve the desired mixture for some commonly used concentrations.
For general anaesthesia, remifentanil is most commonly added to 1% propofol to make a final concentration of 5 micrograms/ml of remifentanil in the propofol. See Table 1 for an example of how to achieve this. Tables 2, 3 and 4 describe how to achieve other concentrations of remifentanil in 1% propofol.
Induction
Maintaining IV access is essential; it is important to hold the patient’s hand while the initial bolus is delivered. This prevents the patient pulling their hand away and dislodging the cannula during induction. As a guide, the pump should initially be programmed to a Cpt of 5 - 6 mcg/ml. For some younger patients (aged 3 and under) this may deliver an inadequate induction bolus and the pump may need to be set higher, such as 7.0mcg/ml. Once the patient is anaesthetised, the Cpt can be reduced to around 3.0mcg/ml (if planning an LMA) or 4.0mcg/ml (if planning to intubate the patient). If, during airway management, it is apparent the patient is not deeply enough anaesthetised, efforts to secure the airway should stop, the TCI increased by approximately 1.0mcg/ml and equilibrium allowed to occur before airway management is resumed.
CAUTION: Using a 22g cannula can result in high syringe pressure at the pump. It is recommended to increase the “high” limit of the pressure alarm on the pump prior to delivering the induction bolus to at least level 8. This is especially important if the cannula tip is sitting at the wrist joint. Consideration should be given to reducing the pressure alarm limit after induction to ensure cannula failure is noticed promptly.
 Figure 4. Button on Alaris PK pump to change pump pressure alarms
Figure 4. Button on Alaris PK pump to change pump pressure alarms
CAUTION: The induction bolus of propofol and remifentanil will take longer to anaesthetise the patient than a manual bolus of propofol, and the patient will desaturate more readily if not pre-oxygenated. As the patient goes off to sleep, it is important to pre-oxygenate.
CAUTION: The bolus of remifentanil will sometimes give the patient a degree of chest wall rigidity. As the induction bolus continues, the propofol will deepen anaesthesia and the rigidity will cease. Knowing this will prevent unnecessary gastric insufflation.
Inhalational Induction
It will sometimes be necessary to commence TIVA after an inhalational induction. What rate the infusion is commenced at will depend on different factors, including:
- Planned airway management technique (LMA/ETT/FM)
- Desire for SV/IPPV
- Time taken to cannulate after induction
- Use of N2O during induction
If wishing to intubate and ventilate, a starting Cp of 4mcg/ml would be an appropriate initial setting.
If wishing to maintain spontaneous ventilantion, a level as low as 2mcg/ml would be appropriate as significant sevoflurane will still be in the system. It is important to titrate Cp up as sevoflurane declines to avoid inadequate depth of anaesthesia.
Neuromuscular Blocking Drugs
As with the use of volatile agents, neuromuscular blockade is not always required. However, the Association of Anaesthetists state (and the authors are in agreement): “Use of a processed EEG monitor is recommended when a neuromuscular blocking drug is used with TIVA.” [1]
Maintenance
As a guide, typical target concentrations (Cpt) for this technique are as follows:
Spontaneous/controlled ventilation (LMA) 2.5mcg/ml – 4.0mcg/ml
Spontaneous ventilation (ETT) 3.5mcg/ml – 6.0mcg/ml
Controlled ventilation (ETT) 4.0mcg/ml – 6.0mcg/ml
Please note that, as with volatile agents, Cp should be titrated for each patient and case individually.
Emergence
Once the infusion has been stopped, the plasma levels of drug will fall independent of respiration, and the patient may start to wake up quickly. It is at the discretion of the anaesthetist whether the airway device is removed deep or awake, but it is worth noting the potential quick wake-up may lead to emergence between theatre and recovery.
Flushing Intravenous Cannulae
All intravenous cannulae MUST be flushed prior to leaving theatre.
The flushing MUST be documented on the anesthetic record.
The flushing MUST be verbally handed over to recovery staff.
This is an advanced technique to be used only when experienced in the use of TIVA in paediatric patients.
There is an advantage to the use of TIVA for MLB procedures and for removal of airway foreign bodies. The separation of oxygenation from anaesthesia in the patient with a partially obstructed airway means an appropriate anaesthetic depth can be achieved without relying on inhaling sevoflurane. However, over-delivery of the propofol/remifentanil mixture will lead to apnoea, and the potential need for bag-mask ventilation. Positive pressure ventilation risks distal displacement of any foreign body.
Preparation
Intravenous access is obtained and pre-oxygenation commenced via facemask. Once pre-oxygenation is complete, general anaesthesia may commence.
Induction
The same propofol/remifentail mixture is used as described in table 1 (1% propofol with 5mcg/ml remifentanil) via an appropriate TIVA giving set.
This is a technique that takes time and the patient and their parent/carer should be warned that induction may take longer than anticipated. The Cpt should be started at 1.0mcg/ml and slowly increased every minute by 1.0 or 0.5, depending on clinical response. During this time it is advisable to hold the patient’s hand to avoid the cannula becoming dislodged by any excitatory movements during induction of general anaesthesia.
Once anaesthesia has reached the desired depth, topical preparation of the nose and vocal cords can be achieved in the usual way and the airway managed in a similar way as if volatile anaesthesia were being used.
Inhalational Induction
This is as described in the previous section but, to ensure spontaneous ventilation, it is necessary to be very cautious with the up-titration of the propofol/remifentanil mixture.
Maintenance
A Cp of approximately 3.5 – 6.0 mcg/ml will be required to maintain anaesthesia during MLB/foreign body extraction. These levels are a guide only and must be titrated to clinical effect, as with volatile anaesthesia.
TIVA can be used for GI endoscopy without the use of an endotracheal tube or LMA. This is a well-established technique in many paediatric centres worldwide with patient and procedural benefits including:
- Reduced recovery time
- Quicker resumption of oral intake
- Improved list turnover/throughput
- All the other benefits of TIVA!
However, careful patient selection is required. Contraindications include:
- Patients with GORD
- Patients with reduced gastric emptying
- Patients with active vomiting
- Some obese patients where oxygenation during this technique can be challenging
- Young patients (authors recommend caution if patient <5yr, low threshold for intubation)
In general, the concentration of remifentanil is less than previously described for general anaesthesia. This helps to maintain spontaneous ventilation in the face of reduced procedural stimulation once the endoscope is inserted.
To achieve a solution of 1% propofol with 2.5 micrograms / ml of remifentanil:
- 1mg of remifentanil is diluted in 20ml 0.9% sodium chloride, giving a concentration of 50 micrograms / ml
- 2.5ml of this remifentanil is added to 47.5ml of 1% propofol
Lidocaine is either added to the pump or directly to the vein after cannulation. This choice is one of personal preference, but the authors do recommend the use of lidocaine to reduce pain on induction.
Induction
Although desirable, IV induction is not essential. If required gas induction can be achieved and then TIVA commenced after cannulation. Care must be taken to ensure depth of anaethesia is maintained as the sevoflurane is washing out and the TIVA is reaching Ce.
If induction is IV, commence infusion at a Cpt of 3.0 mcg/ml and assess response. In older children you may have to titrate to 6-7 mcg/ml before loss of verbal contact.
Apply a gentle jaw thrust and assess response: increase Cpt by 0.2 mcg/ml until jaw thrust tolerated and patient has a stable respiratory pattern. General guide is to aim for a respiratory rate half of normal baseline in younger children or <15/min in adolescents.
How to oxygenate
Preference is to use the anaesthetic face mask initially, but once the patient is asleep with a stable respiratory rate, high flow nasal cannulae are applied and flow commenced as per HFNO guide (attached to Optiflow machine).
Keep the face mask handy and discuss with your assistant the size of ETT you will require should your airway management plan change during the case.
Patients may require basic airway maneouvres if evidence of obstruction occurs. Insertion of a bite block should be considered to protect the endoscope.
Maintenance
Keep IV access visible and accessible and the TIVA pump near you. Open the airway for the OGD. If there is evidence of insufficient depth of anaesthesia increase Cpt by 0.2mcg/ml and allow equilibrium. Placing the face mask on the chest/abdomen allows for improved visualisation of the respiratory pattern and ensures you know where it is should you require it quickly!
Emergence
Continue to oxygenate the patient and transfer to recovery.
All intravenous cannulae MUST be flushed prior to leaving theatre.
The flushing MUST be documented on the anesthetic record.
The flushing MUST be verbally handed over to recovery staff.
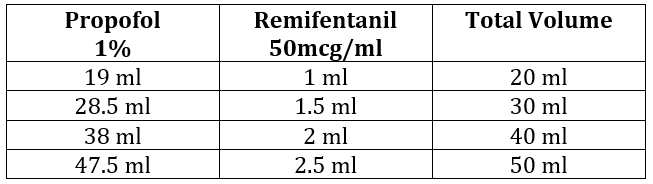
Table 1: To make remifentanil concentration of 5mcg/ml in 1% Propofol (with lidocaine added to the syringe)
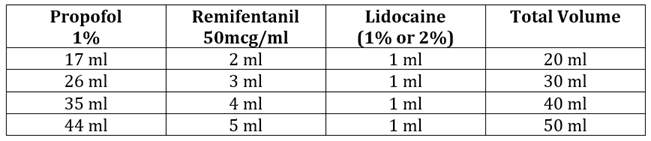
Table 2: To make remifentanil concentration of 5mcg/ml in 1% Propofol (without lidocaine added to the syringe)

Table 3: To make remifentanil concentration of 2.5mcg/ml in 1% Propofol (with lidocaine added to the syringe)
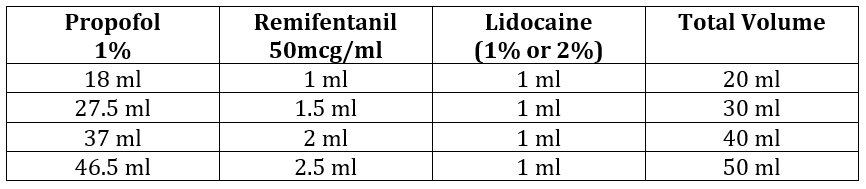
Table 4: To make remifentanil concentration of 2.5mcg/ml in 1% Propofol (without lidocaine added to the syringe)
PRIS is a rare multi-organ disorder which has a high mortality (~50%) once established. It is related to the use of propofol and must be suspected in patients who become unwell during the use of propofol infusions, particularly if no other cause can be established.
Risk factors include prolonged infusion, high delivery rates (>6mg/kg/hour), high cumulative dose, concurrent critical illness, low sugar intake and co-administration of catecholamines and steroids.
The commonest presentation is metabolic acidosis, with ECG changes the second commonest presentation. Other presenting features are listed below in table 5.
Treatment strategies involve stopping the infusion of the drug, treating organ dysfunction symptomatically and encouraging excretion of propofol and its’ metabolites from the body. Haemofiltration and extra-corporeal membrane oxygenation have both been used with success in the treatment of PRIS.
Table 5. Symptoms and Signs of PRIS
|
System Involvement |
Symptoms / Signs |
|---|---|
|
Cardiac |
Cardiac failure including pulmonary oedema |
|
Vascular |
Hypotension |
|
Renal & Urinary |
Acute Kidney Injury |
|
Musculoskeletal & Connective Tissue |
Rhabdomyolysis |
|
Metabolism & Nutrition |
Metabolic acidosis |
|
Hepatobiliary |
Hepatomegaly |
- Nimmo AF, Absalom AR, Bagshaw O, Biswas A et al. Safe Practice of Total Intravenous Anaesthesia. Joint guidelines from the Association of Anaesthetists and the Society for Intravenous Anaesthesia (TIVA) 2018
- Bagshaw O, McCormack J, Brooks P, Marriott D, Baxter A. The safety profile and effectiveness of propofol-remifentanil mixtures for total intravenous anaesthesia in children. Paediatr Anaesth. 2020 Dec;30(12):1331-1339
- Hemphill S, McMenamin L, Bellamy M, Hopkins P. Propofol infusion syndrome: a structured literature review and analysis of published case reports. BJA 2019; 122(4): 448-459
Further Reading
- TOTW: A Practical Approach to Propofol-Based Total Intravenous Anaesthesia (TIVA) in Children, Nov 2018
- Gaynor J, Ansermino JM. Paediatric Total Intravenous Anaesthesia. BJA Education November 2016; 16 (11): 369–373
- Malherbe S, Barker N. Mixing of propofol and remifentanil. Paediatr Anaesth, 2021;31:504-505
- Allison E, Malherbe S, Barker N. Drug mixtures and infusion technology. Anaesthesia. 2022 Jl;77(7):835
Last reviewed: 30 September 2023
Next review: 30 September 2025
Author(s): Dr Jocelyn Erskine & Dr Neal Willis
Approved By: Department of Anaesthesia

